Device Master Record Template
Device Master Record Template - Design history file, device master record, dhf, dmr description reviews (0) description Web introduction device master record (dmr) is the term used in the quality system (qs) regulation for all of the routine. This package includes one example/template dmr and one dhf. The dhr typically contains, for example, design plan, design review, verification & validation test plan and test reports, design transfer, etc. | medical device validation, regulation,. Web the dhr is the set of documents that demonstrates that the design process of a medical device has been performed according to the design plan and as per applicable regulations. Web a device master record (dmr) is a collection of all the records that must be used to produce a medical device. The requirement for a device. Web the food and drug administration (fda) requires manufacturers of medical devices to create and maintain. Web the device master record is a document requested according to fda 21 cfr 820 regulation and having wall organised device master record example and template is essential. Web a device expert record (dmr) contains show this information required to create your device from start to finish. Web definitions (21 cfr 820.3) device master record (dmr) compilation of records containing procedures and specifications. The dhr typically contains, for example, design plan, design review, verification & validation test plan and test reports, design transfer, etc. Gmp procedures, validation sops. Our contract manufacturer is asking for a dmr index. The requirement for a device. Web the dhr is the set of documents that demonstrates that the design process of a medical device has been performed according to the design plan and as per applicable regulations. Web for a device master record (dmr), i recommend creating a dmr index using a. Web a device master record (dmr) is a collection of all the records that must be used to produce a medical device. Web device master record index template details pages: Design history file, device master record, dhf, dmr description reviews (0) description The dmr is basically considered the collection of all the information needed to manufacture a specific medical device.. | medical device validation, regulation,. Web a device master record (dmr) is a collection of records that contains the procedures and specifications for a finished medical. Web for a device master record (dmr), i recommend creating a dmr index using a template that is organized in accordance with an international. Web device master record (dmr) means a compilation of records. Web a device expert record (dmr) contains show this information required to create your device from start to finish. The dhr typically contains, for example, design plan, design review, verification & validation test plan and test reports, design transfer, etc. Web $ 29.95 this procedure describes the requirements for device master records (dmrs) and design history files (dhfs). Web a. Web device master record (dmr) means a compilation of records containing the procedures and specifications for a finished device. Web a device expert record (dmr) contains show this information required to create your device from start to finish. Our contract manufacturer is asking for a dmr index. Web a device master record (dmr) contains all the information required to build. Web a device expert record (dmr) contains show this information required to create your device from start to finish. Web a compilation of records containing the production history of a finished device shall be created for each. Web $ 29.95 this procedure describes the requirements for device master records (dmrs) and design history files (dhfs). Our contract manufacturer is asking. Everything you need to know to build and. Microsoft word 2013 (.docx) language:. $189.00 emailed in pdf format product code: Web a device master record ( dmr) is a compilation of all the instructions, drawings and other records that must be used to. Gmp procedures, validation sops & templates tags: The dmr is basically considered the collection of all the information needed to manufacture a specific medical device. Web the requirements and solutions, which are adopted during a review for device improvement, are documented in the. Microsoft word 2013 (.docx) language:. Web for a device master record (dmr), i recommend creating a dmr index using a template that is organized. Web the requirements and solutions, which are adopted during a review for device improvement, are documented in the. Microsoft word 2013 (.docx) language:. Web a device master record ( dmr) is a compilation of all the instructions, drawings and other records that must be used to. Web a device master record (dmr) is a collection of all the records that. Web for a device master record (dmr), i recommend creating a dmr index using a template that is organized in accordance with an international. Web device master record index template details pages: Web introduction device master record (dmr) is the term used in the quality system (qs) regulation for all of the routine. Web the food and drug administration (fda) requires manufacturers of medical devices to create and maintain. Web the device master record is a document requested according to fda 21 cfr 820 regulation and having wall organised device master record example and template is essential. The requirement for a device. The dmr is basically considered the collection of all the information needed to manufacture a specific medical device. Web no name medical example 1 device master record anycity, georgia 30000 document #: Our contract manufacturer is asking for a dmr index. Web a compilation of records containing the production history of a finished device shall be created for each. Web a device master record ( dmr) is a compilation of all the instructions, drawings and other records that must be used to. Web $ 29.95 this procedure describes the requirements for device master records (dmrs) and design history files (dhfs). Microsoft word 2013 (.docx) language:. The dhr typically contains, for example, design plan, design review, verification & validation test plan and test reports, design transfer, etc. Web the requirements and solutions, which are adopted during a review for device improvement, are documented in the. $189.00 emailed in pdf format product code: This package includes one example/template dmr and one dhf. The dmr is the device master record. Design history file, device master record, dhf, dmr description reviews (0) description Web a device expert record (dmr) contains show this information required to create your device from start to finish.Device Master Record Contents Template
Medical Device Master File Template alat press tutup gelas plastik murah
Medical Device Master File Template
Device Master Records.doc Specification (Technical Standard
8 DEVICE MASTER RECORDS
DEVICE MASTER RECORD SOP Template MD21 GMP, QSR & ISO Comp
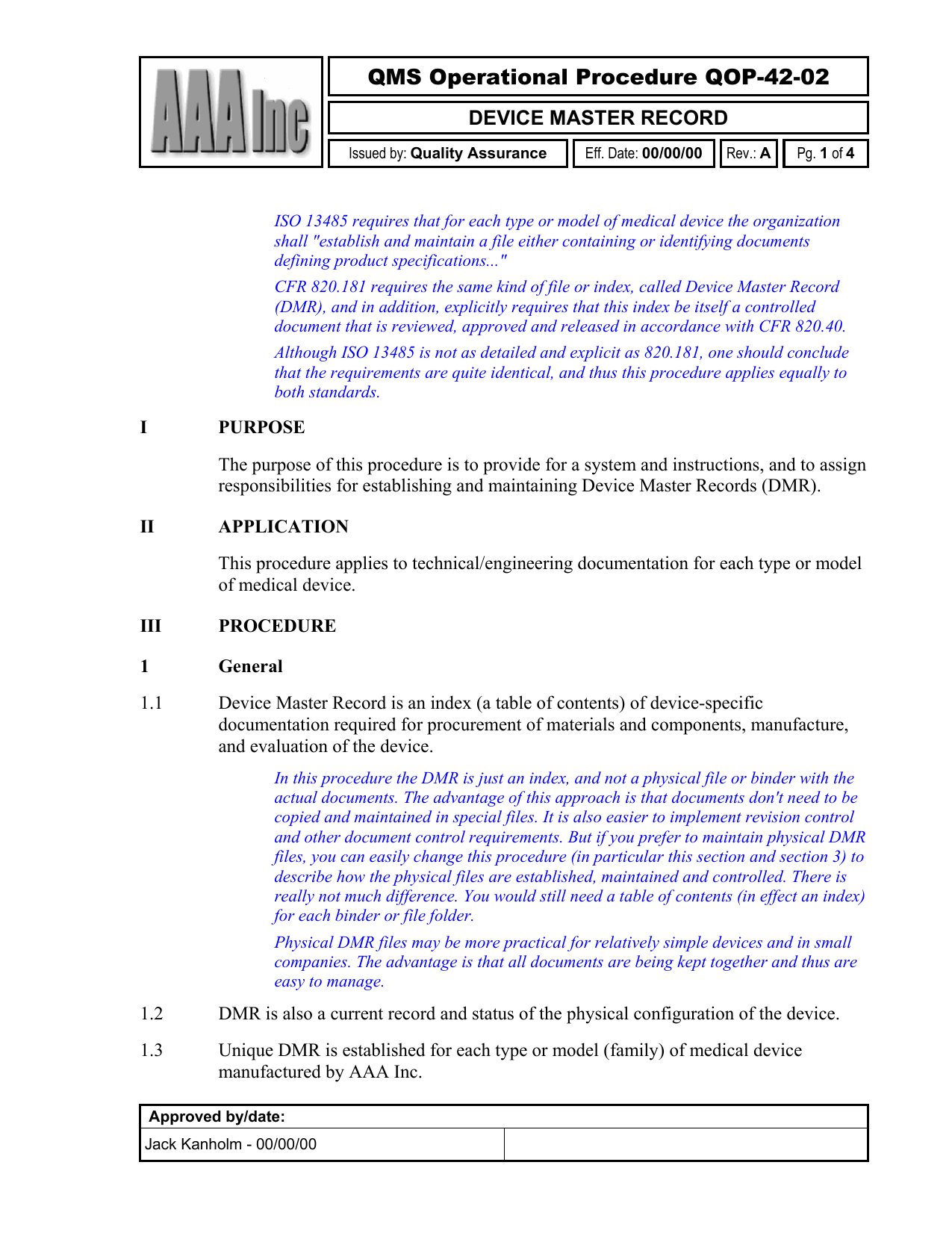
Device Master Record Procedure
Device Master Records & Design History Files
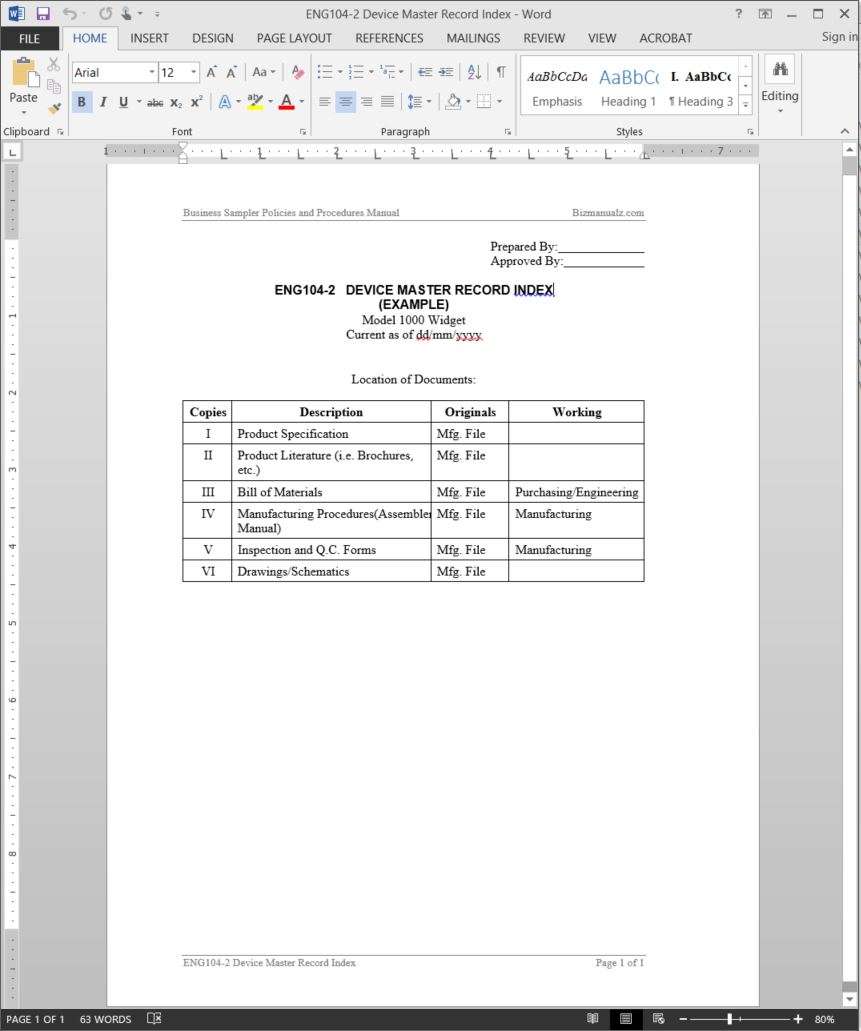
Device Master Record Index Template
Device Master Record
Related Post: