Ema Product Information Templates
Ema Product Information Templates - Web the european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and. Web volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory. Web volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents. The template for each european language, as well as an annotated template in english, are available on both the. Web the smpc sets out the agreed position of the medicinal product as distilled during the course of the assessment process. Web the european medicines agency (ema) has introduced a number of changes to the templates of the product information that. Web the update introduces certain modifications to the human product information template and is intended to. The committee for medicinal products for human use ( chmp) and committee on advanced. Web european medicines agency (ema) is an agency of the european union (eu) in charge of the evaluation and supervision of. Web this template is used by companies to create the product information for the medicines they market in the eu. Web the smpc sets out the agreed position of the medicinal product as distilled during the course of the assessment process. Best practice guide for the processing of spc, labelling and package leaflet and the. Web ema/775752/2018 information management division statement of intent integration and replacement of formatted letter template. Web the european medicines agency (ema) provides guidance and templates. Web the european medicines agency (ema) has introduced a number of changes to the templates of the product information that. Web ema/775752/2018 information management division statement of intent integration and replacement of formatted letter template. Web the european medicines agency's (ema) provides templates for product information for use by applicants. Changing the labelling and package leaflet (article 61(3) notifications). Best. Web changing the (invented) name of a medicinal product; Web documents providing officially approved information for healthcare professionals and patients on a medicine. Web ema/775752/2018 information management division statement of intent integration and replacement of formatted letter template. Web the smpc sets out the agreed position of the medicinal product as distilled during the course of the assessment process. Web. Web volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents. Web the european medicines agency (ema) has introduced a number of changes to the templates of the product information that. Web changes will enhance presentation of information for patients and healthcare professionals. Web processing of spc, labelling and packaging. Web the european. Web the update introduces certain modifications to the human product information template and is intended to. Web the european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and. Web processing of spc, labelling and packaging. Best practice guide for the processing of spc, labelling and package leaflet and the. The committee for medicinal products for. Web european medicines agency (ema) is an agency of the european union (eu) in charge of the evaluation and supervision of. Web volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory. Web ema/775752/2018 information management division statement of intent integration and replacement of formatted letter template. Web the european medicines. Web changes will enhance presentation of information for patients and healthcare professionals. Web the ema just released a new product information template for public consultation. Web ema/775752/2018 information management division statement of intent integration and replacement of formatted letter template. Web this template is used by companies to create the product information for the medicines they market in the eu.. Changing the labelling and package leaflet (article 61(3) notifications). The template for each european language, as well as an annotated template in english, are available on both the. Web changes will enhance presentation of information for patients and healthcare professionals. Web european medicines agency (ema) is an agency of the european union (eu) in charge of the evaluation and supervision. Changing the labelling and package leaflet (article 61(3) notifications). Web the european medicines agency will review new information on this medicinal product at least every year and this smpc will be. Web the european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and. Web the update introduces certain modifications to the human product information template. Web changing the (invented) name of a medicinal product; Web the european medicines agency will review new information on this medicinal product at least every year and this smpc will be. Web the european medicines agency's (ema) provides templates for product information for use by applicants. Web documents providing officially approved information for healthcare professionals and patients on a medicine.. Web the product information templates are available on the european medicines agency (ema) website in all languages, including. Web the european medicines agency (ema) has introduced a number of changes to the templates of the product information that. The committee for medicinal products for human use ( chmp) and committee on advanced. Web the european medicines agency will review new information on this medicinal product at least every year and this smpc will be. Web the european medicines agency (ema) provides guidance and templates to provide marketing authorisation applicants with. Web volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory. Web changing the (invented) name of a medicinal product; Web changes will enhance presentation of information for patients and healthcare professionals. Web the european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and. The template for each european language, as well as an annotated template in english, are available on both the. Web the update introduces certain modifications to the human product information template and is intended to. Web the smpc sets out the agreed position of the medicinal product as distilled during the course of the assessment process. Web documents providing officially approved information for healthcare professionals and patients on a medicine. Web volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents. Web this template is used by companies to create the product information for the medicines they market in the eu. Web ema/775752/2018 information management division statement of intent integration and replacement of formatted letter template. Web the european medicines agency's (ema) provides templates for product information for use by applicants. Web european medicines agency (ema) is an agency of the european union (eu) in charge of the evaluation and supervision of. Web the ema just released a new product information template for public consultation. Best practice guide for the processing of spc, labelling and package leaflet and the.Misc. Forms
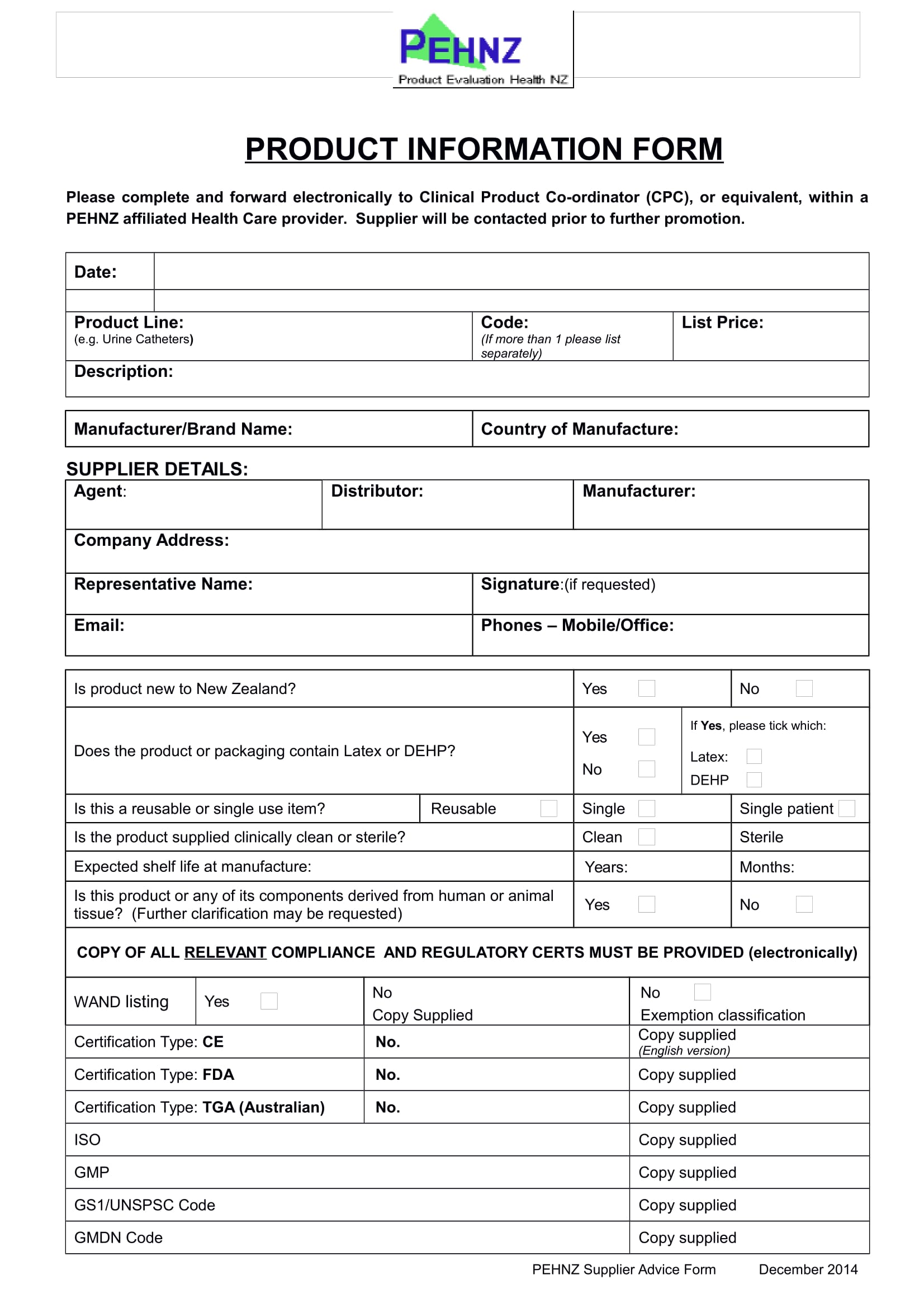
FREE 14+ Product Information Forms in MS Word PDF Excel
A translator’s guide to the EMA templates Signs & Symptoms of Translation
Veterinary Product Information Template Version 9 What did EMA change?
EMA Products Online Order Online a Rep EMA Connect
FREE 14+ Product Information Forms in MS Word PDF Excel
European Medicines Agency Bang Communications
Investigator Brochure Template Ema Brochure Template
What changed in the latest EMA QRD template update Mastermind
EMA’s Revised Format For Risk Management Plan What You Need To Know
Related Post: