Fda Label Template
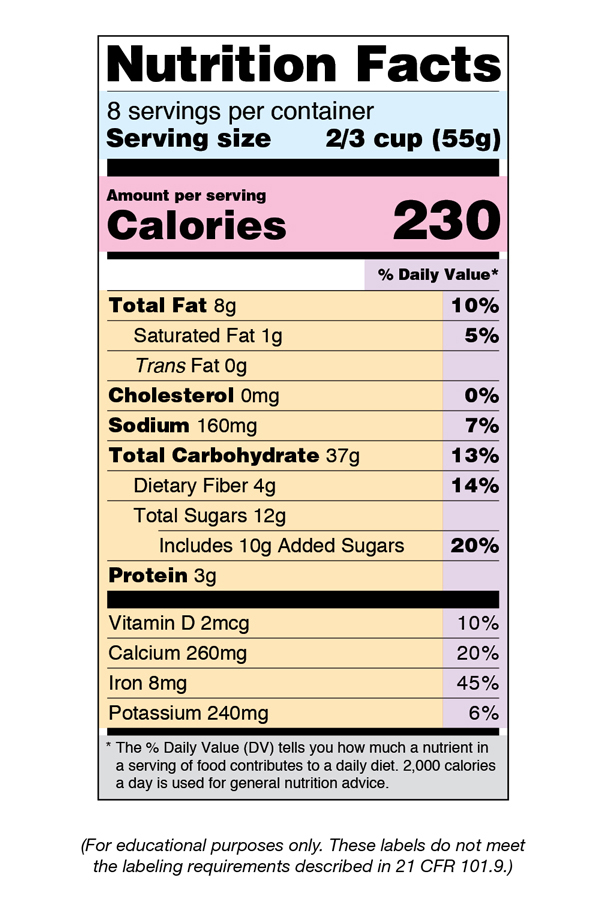
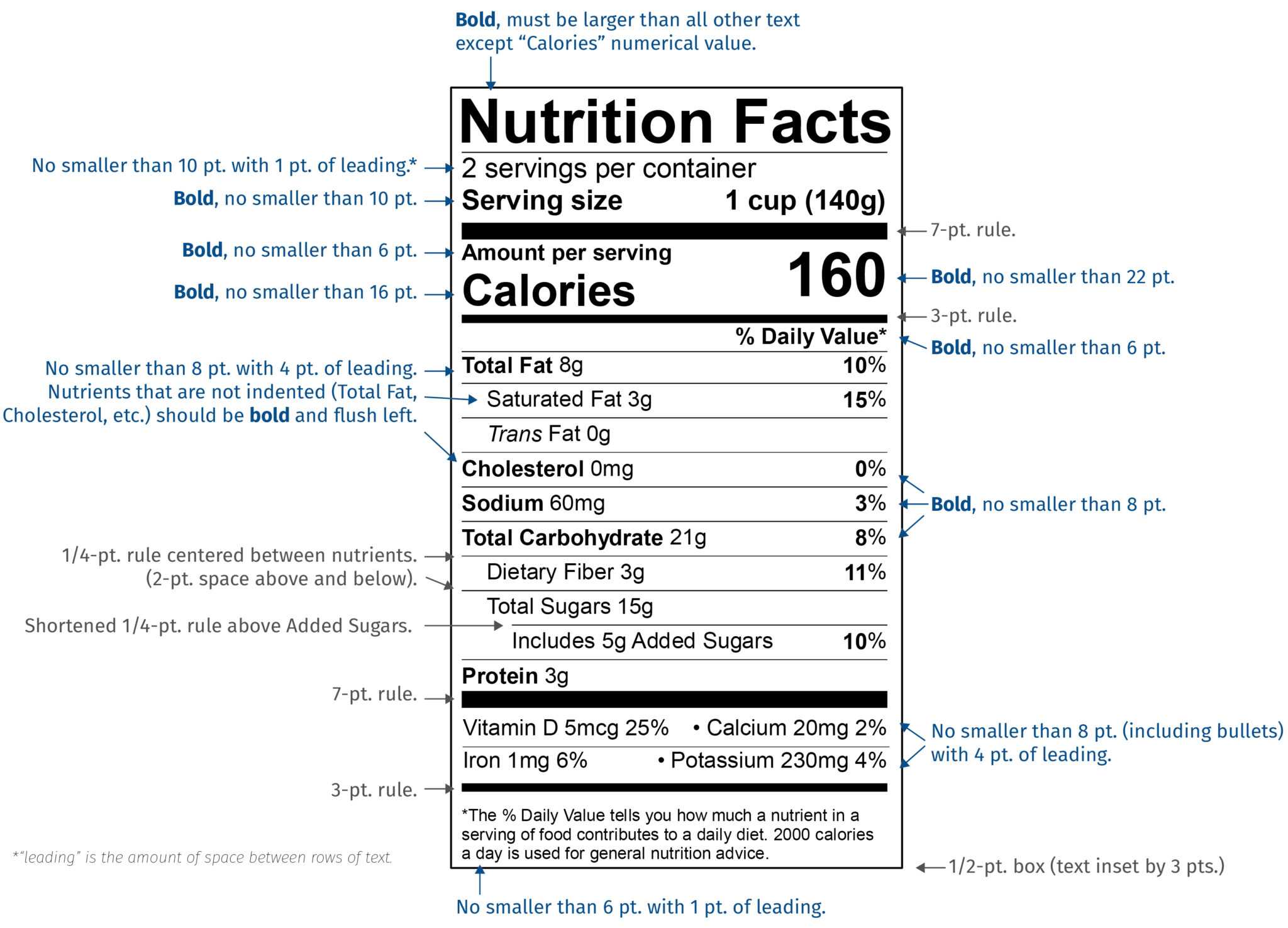
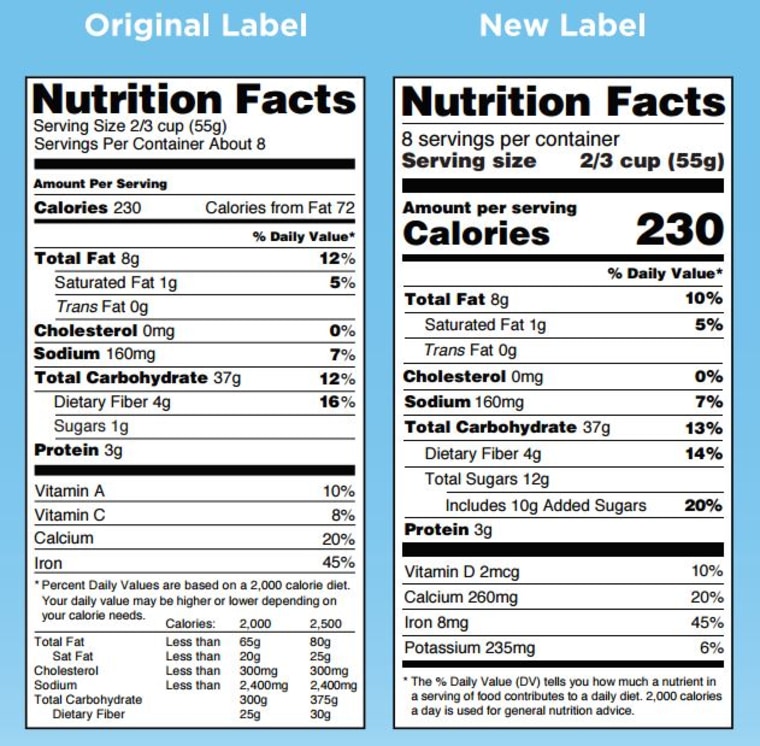
Fda Label Template - Web search by active ingredient: Web for more information on labeling, including physician labeling rule (plr) requirements, guidances,. Web in labeling, in accordance with regulatory requirements for the content and format of human prescription drug labeling. Human rx or choose one or more from the list: Example formats available in this. Changes to the nutrition facts label; Name and address of distributor specific container label statement requirements 21 cfr 201.50: Web their appropriate product label. Dmf holder name change letter. Web renvela >5.5 and <7.5 mg/dl ; One suboxone® (buprenorphine and naloxone) 8 mg/2 mg sublingual tablet provides. 1.6 g three times daily with meals : Web renvela >5.5 and <7.5 mg/dl ; (type in part or all of active ingredient) return to the fda label search page. Web food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts,. 0.8 g three times daily with meals 7.5 mg/dl ; Web renvela >5.5 and <7.5 mg/dl ; Web in labeling, in accordance with regulatory requirements for the content and format of human prescription drug labeling. Web fdalabel restore last query labeling types choose one or more: Web for more information on labeling, including physician labeling rule (plr) requirements, guidances,. One suboxone® (buprenorphine and naloxone) 8 mg/2 mg sublingual tablet provides. Web <50 x 109/l following at least 2 weeks of promacta. Nutrition facts labels you are here: Web examples of different label formats that using the new nutrition facts label. Web in labeling, in accordance with regulatory requirements for the content and format of human prescription drug labeling. Nutrition facts labels you are here: Web search for labels on dailymed. ≥200 x 109/l to ≤400 x 109/l at any time. Web renvela >5.5 and <7.5 mg/dl ; Web 21 cfr 610.64: Web food safety plan & haccp templates. Home / esha products / genesis r&d. The labels are also available on the national library of medicine's dailymed web site. Web fda’s nutrition initiatives; Web their appropriate product label. Increase daily dose by 25 mg to a maximum of 75 mg/day. Web fdalabel restore last query labeling types choose one or more: Web 21 cfr 610.64: 0.8 g three times daily with meals 7.5 mg/dl ; Web food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Web for more information on labeling, including physician labeling rule (plr) requirements, guidances,. Web search by active ingredient: Web labelcalc has all of the various nutrition facts label templates built right into the software. Web examples of different label formats that using the new nutrition facts label. Nutrition facts labels you are here: Dmf holder name change letter. Web search by active ingredient: Web their appropriate product label. Web food safety plan & haccp templates. Web fdalabel restore last query labeling types choose one or more: Web examples of different label formats that using the new nutrition facts label. Web food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. The labels are also available on the national library of medicine's dailymed web site. Home / esha products / genesis r&d. ≥200 x 109/l to ≤400. Web food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Web search for labels on dailymed. Dmf holder name change letter. Web in labeling, in accordance with regulatory requirements for the content and format of human prescription drug labeling. Web labelcalc has all of the various nutrition facts label. Web format tools and sample templates general labeling presentations publications prescribing information. (type in part or all of active ingredient) return to the fda label search page. Web our fda nutrition label templates ensures you're in line with fda guidelines. Label claims for food & dietary supplements; Web food safety plan & haccp templates. Web 21 cfr 610.64: Web fda’s nutrition initiatives; One suboxone® (buprenorphine and naloxone) 8 mg/2 mg sublingual tablet provides. 1.6 g three times daily with meals : Here, we show both the current. Web food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Web labelcalc has all of the various nutrition facts label templates built right into the software. Changes to the nutrition facts label; Web renvela >5.5 and <7.5 mg/dl ; Name and address of distributor specific container label statement requirements 21 cfr 201.50: Web us nutrition facts labels & templates | fda food labeling | esha u.s. Human rx or choose one or more from the list: ≥200 x 109/l to ≤400 x 109/l at any time. Increase daily dose by 25 mg to a maximum of 75 mg/day. Web examples of different label formats that using the new nutrition facts label.What You Need to Know About the New FDA Nutrition Fact Label
FDA finalizes new food nutrition labels Vital Record
Point 74 “FDA Approved” Let’s Get Real Pat McCarthy The Point
Nutrition Facts Label Images for Download FDA
New Fda Nutrition Facts Label Font Style And Size Esha for Nutrition
Three steps to plan for the FDA’s new food label rules 20161018
The Easiest Way to Update Your Nutritional Facts Label FlexPAC
Project Body Smart FDA’s New Food Labels What to Know
FDA's New Food Labels What to Know
Nutrition Facts FDA format Inkable Label Co.
Related Post: