Fda Protocol Template
Fda Protocol Template - Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of. Web 20 drug administration (fda) and sponsors or applicants relating to the development and review 21 of drug or biological drug. Web this page includes seven different protocol templates for developing a variety of different new research protocols. The electronic protocol writing tool aims to facilitate the development of. The draft guidance has been. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data. Web 138 rows clinical trials guidance documents. Web this protocol template aims to facilitate the development of two types of clinical trials involving human. Format and content of a rems document: Center for drug evaluation and research, office of regulatory policy this template. Format and content of a rems document: Web 138 rows clinical trials guidance documents. The national institutes of health (nih) and food and drug administration (fda) developed a clinical. Web clinical trial protocols should include a clear description of trial design and patient selection criteria as well as description of. Web 15 rows comparison of fda, epa, oecd glp protocol. Web fda updates the clinical protocol template. Web 15 rows comparison of fda, epa, oecd glp protocol & conduct; Web center for drug evaluation and research mapp 5220.8 rev. Web to set this template's initial visibility, the |state= parameter may be used: Web 20 drug administration (fda) and sponsors or applicants relating to the development and review 21 of drug. Web click the thumbnail to access a free template. Web to set this template's initial visibility, the |state= parameter may be used: Web the fda staff responsible for this guidance as listed on the title page. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of. Web. Web the fda staff responsible for this guidance as listed on the title page. Format and content of a rems document: Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of. Office of generic drugs and office of. Web fda updates the clinical protocol template. (thursday, january 19, 2023) the fda recently released an. The national institutes of health (nih) and food and drug administration (fda) developed a clinical. Web developed jointly by nih and the food and drug administration (fda), the protocol template provides a standard. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded. Office of generic drugs and office of. The national institutes of health (nih) and food and drug administration (fda) developed a clinical. Web 138 rows clinical trials guidance documents. Web this protocol template aims to facilitate the development of two types of clinical trials involving human. Web center for drug evaluation and research mapp 5220.8 rev. Web this page includes seven different protocol templates for developing a variety of different new research protocols. Guidance documents listed below represent the agency's. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of. Web 138 rows clinical trials guidance documents. The draft guidance has been. (thursday, january 19, 2023) the fda recently released an. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national. Web fda updates the clinical protocol template. Web clinical trial protocols should include a clear description of trial design and patient selection criteria as well as description of. Web 138. Web developed jointly by nih and the food and drug administration (fda), the protocol template provides a standard. The draft guidance has been. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data. Web to set this template's initial visibility, the |state= parameter may be used: Guidance documents listed below represent the agency's. Web developed jointly by nih and the food and drug administration (fda), the protocol template provides a standard. Office of generic drugs and office of. Web clinical trial protocols should include a clear description of trial design and patient selection criteria as well as description of. Web 20 drug administration (fda) and sponsors or applicants relating to the development and. Web 15 rows comparison of fda, epa, oecd glp protocol & conduct; Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national. Format and content of a rems document: Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of. Web fda updates the clinical protocol template. Web center for drug evaluation and research mapp 5220.8 rev. Guidance documents listed below represent the agency's. Center for drug evaluation and research, office of regulatory policy this template. The electronic protocol writing tool aims to facilitate the development of. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data. Web this page includes seven different protocol templates for developing a variety of different new research protocols. The national institutes of health (nih) and food and drug administration (fda) developed a clinical. Web to set this template's initial visibility, the |state= parameter may be used: Web 138 rows clinical trials guidance documents. Protocol concurrence will be issued solely based upon the information you provide in the qbr template. (thursday, january 19, 2023) the fda recently released an. Web the fda staff responsible for this guidance as listed on the title page. The draft guidance has been. Web developed jointly by nih and the food and drug administration (fda), the protocol template provides a standard. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of.Protocol Template 05Feb2016 508 Clinical Trial Food And Drug
FDA Software Validation 2022 Guide, Checklist & Template
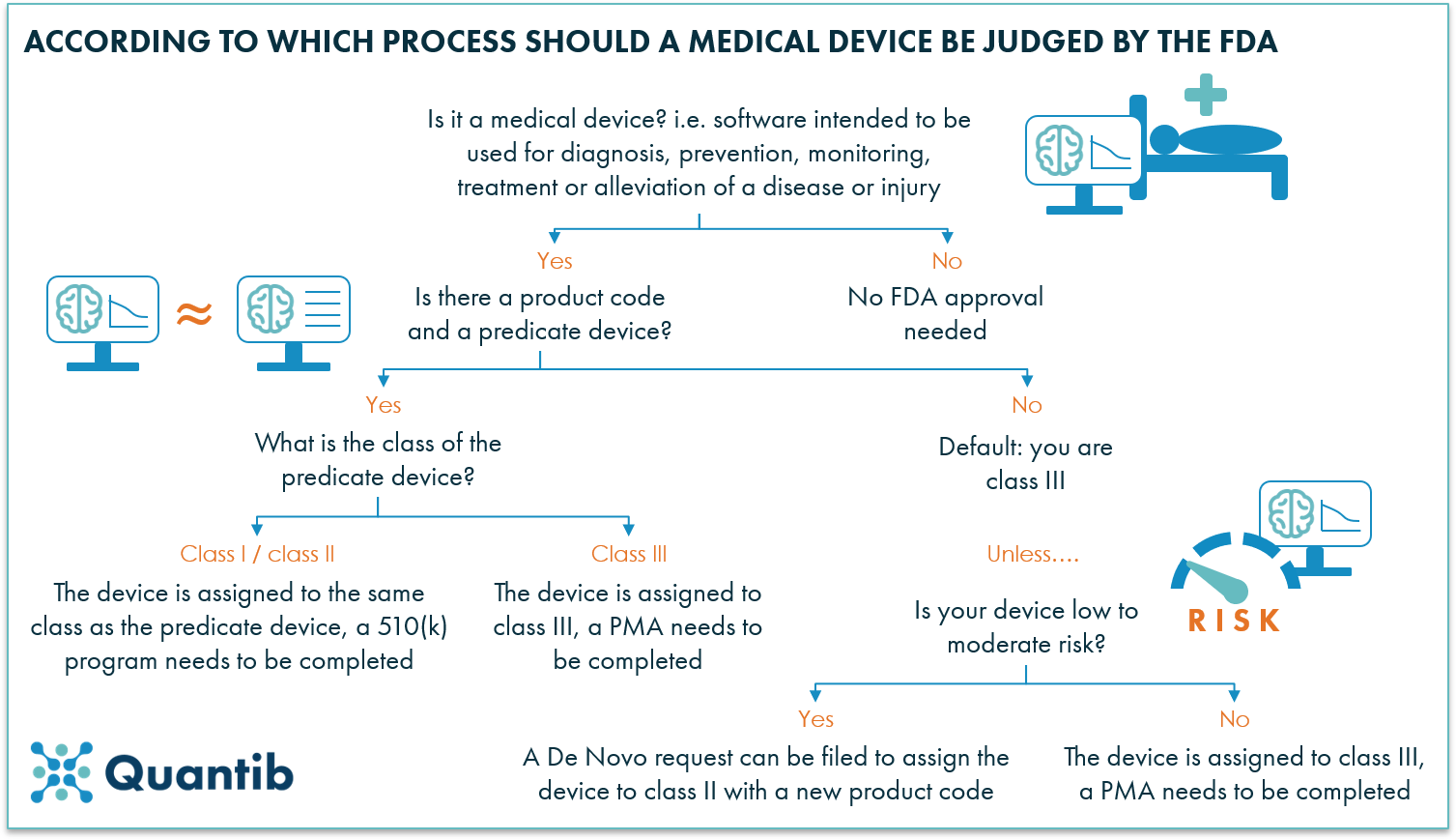
A 101 guide to the FDA regulatory process for AI radiology software
Stability Study Protocol Template williamsonga.us
Iq Oq Pq Software Validation Templates
Three steps to plan for the FDA’s new food label rules 20161018
Clinical Trial Protocol
Validation Master Plan FDA EU WHO Pharma Meddevice Bio
PROCESS VALIDATION SOP Template MD46 GMP, QSR & ISO Compliance
Retail policy and procedure manual template
Related Post: