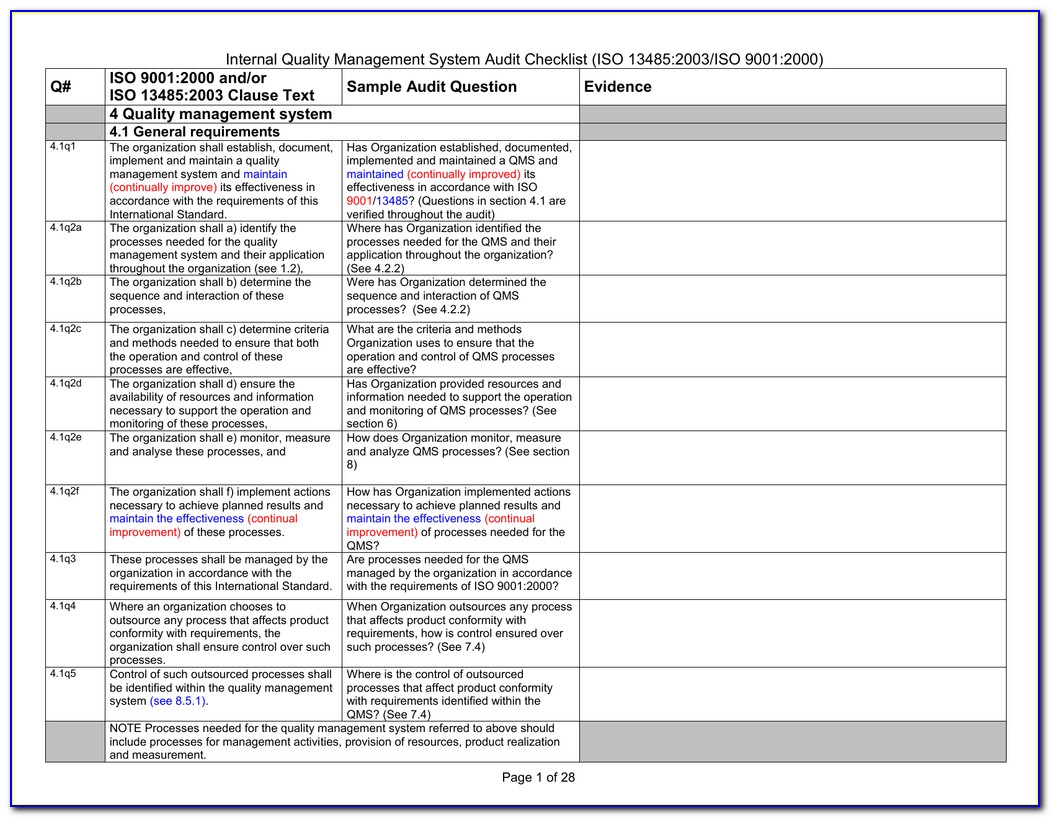
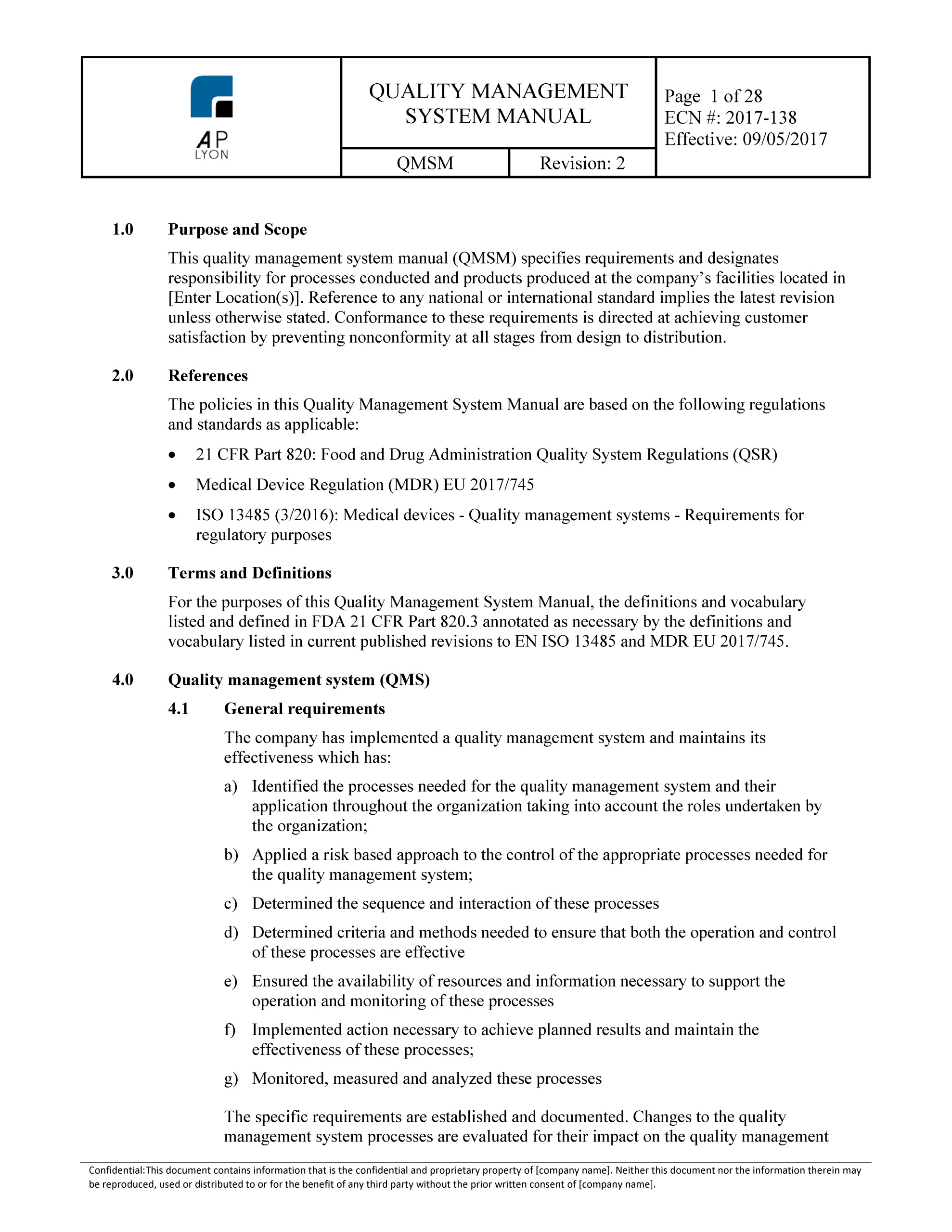
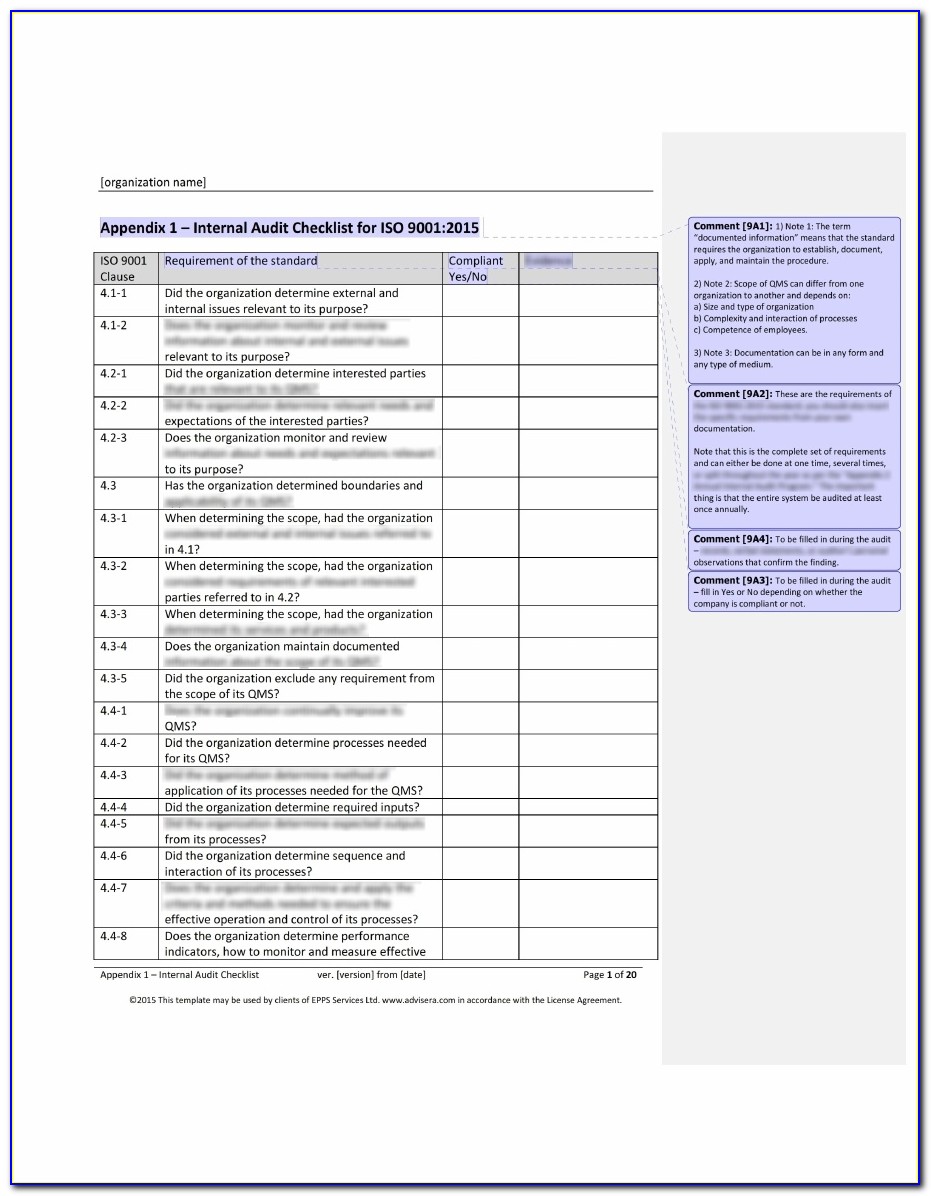
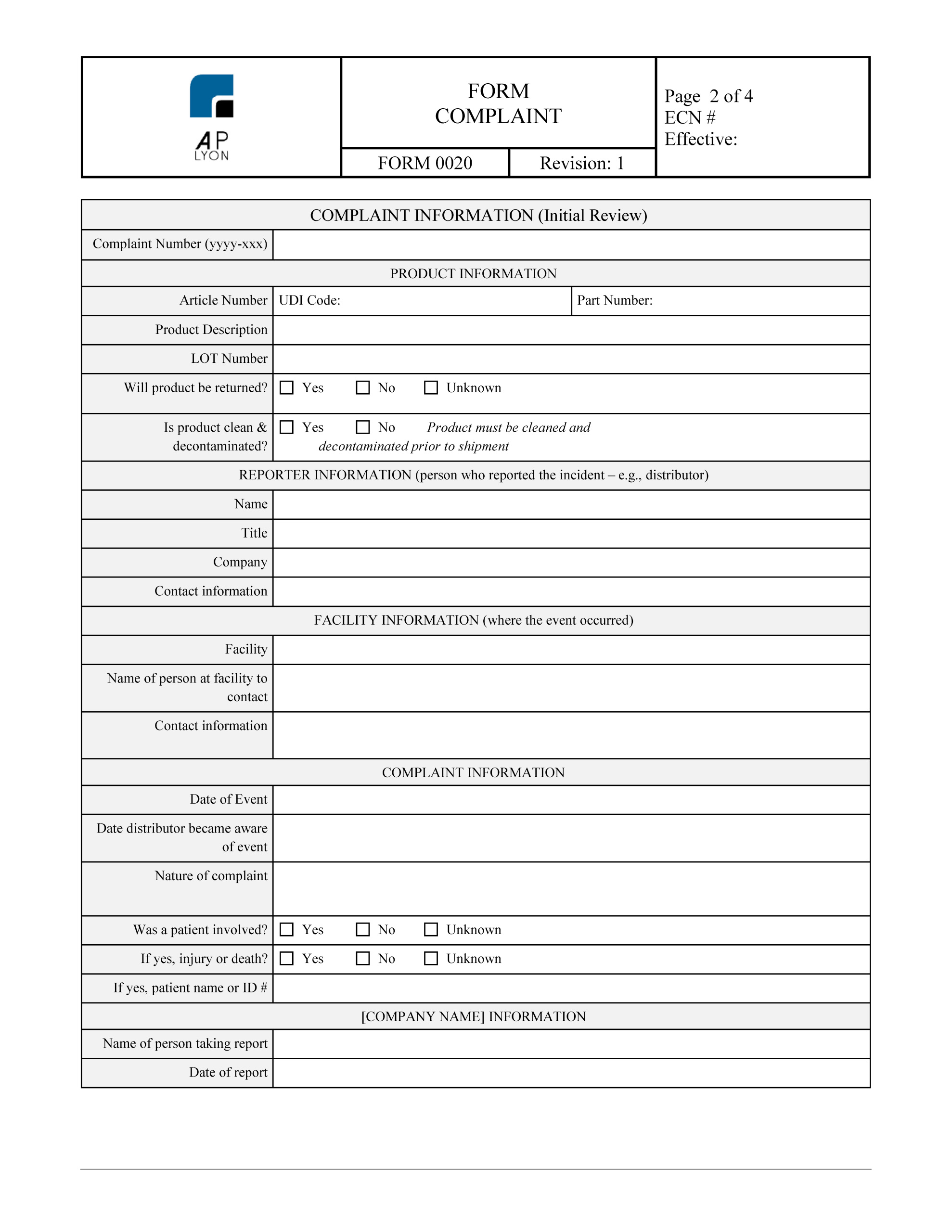
Iso 13485 Templates
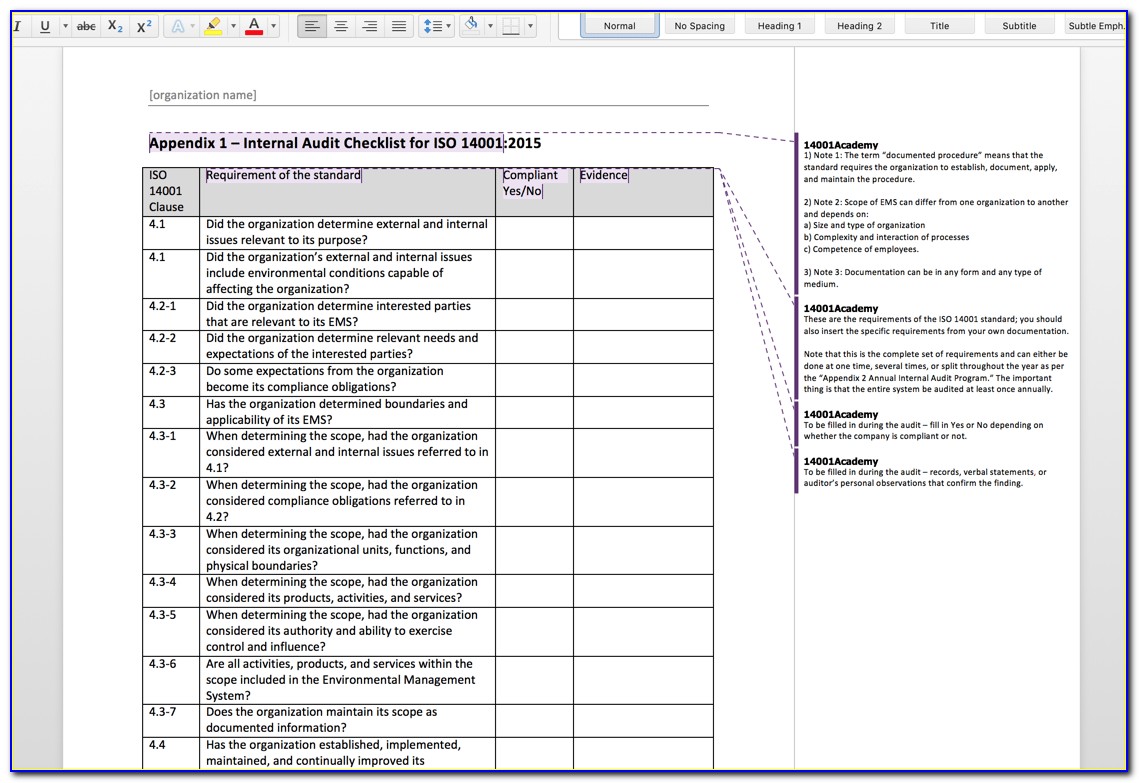
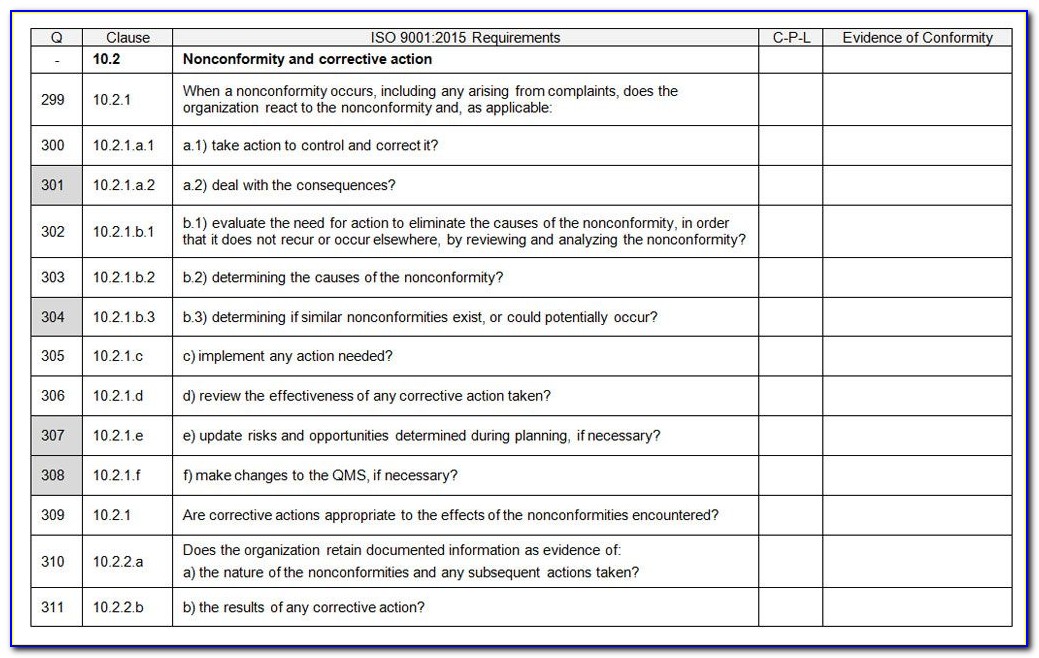
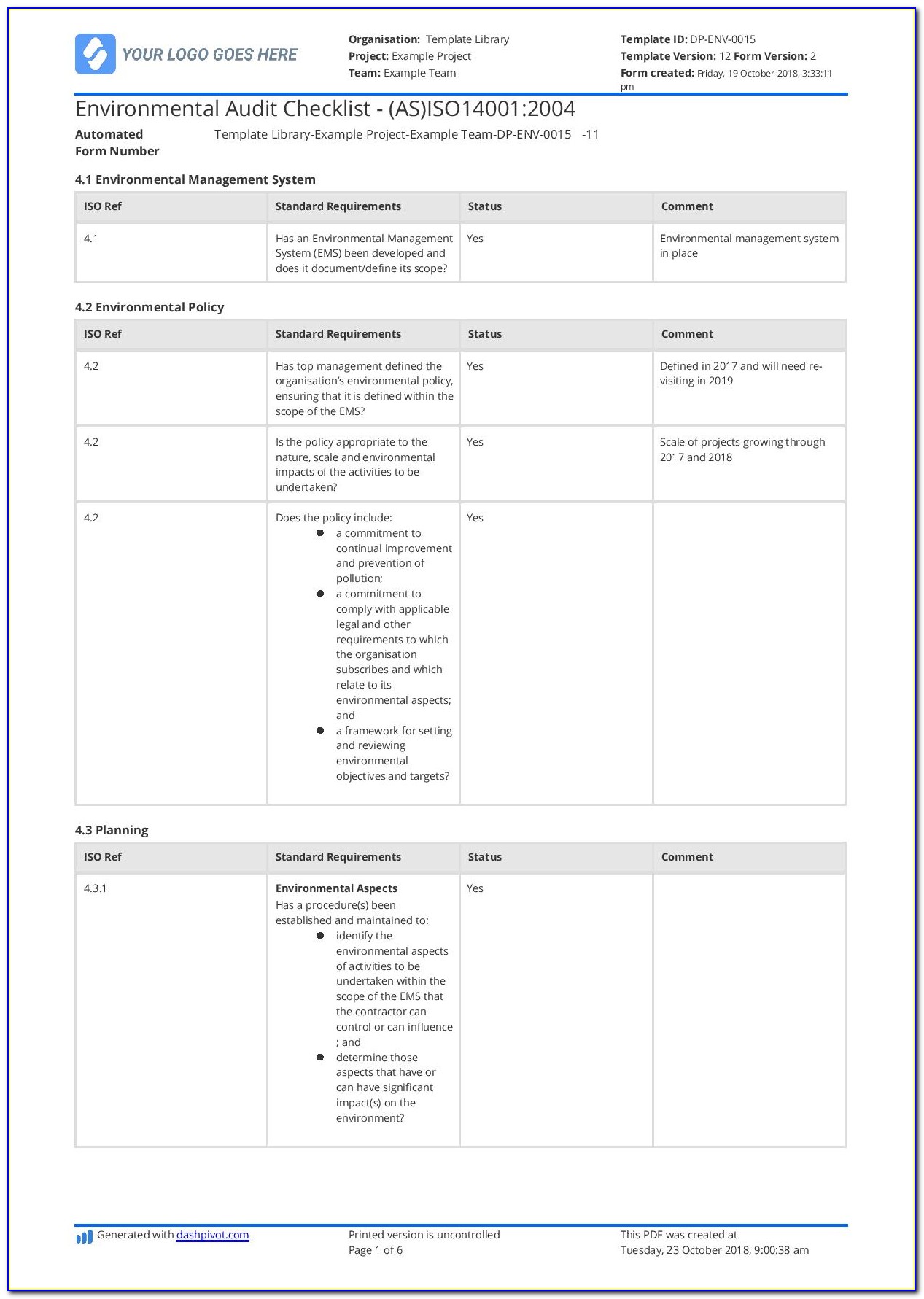
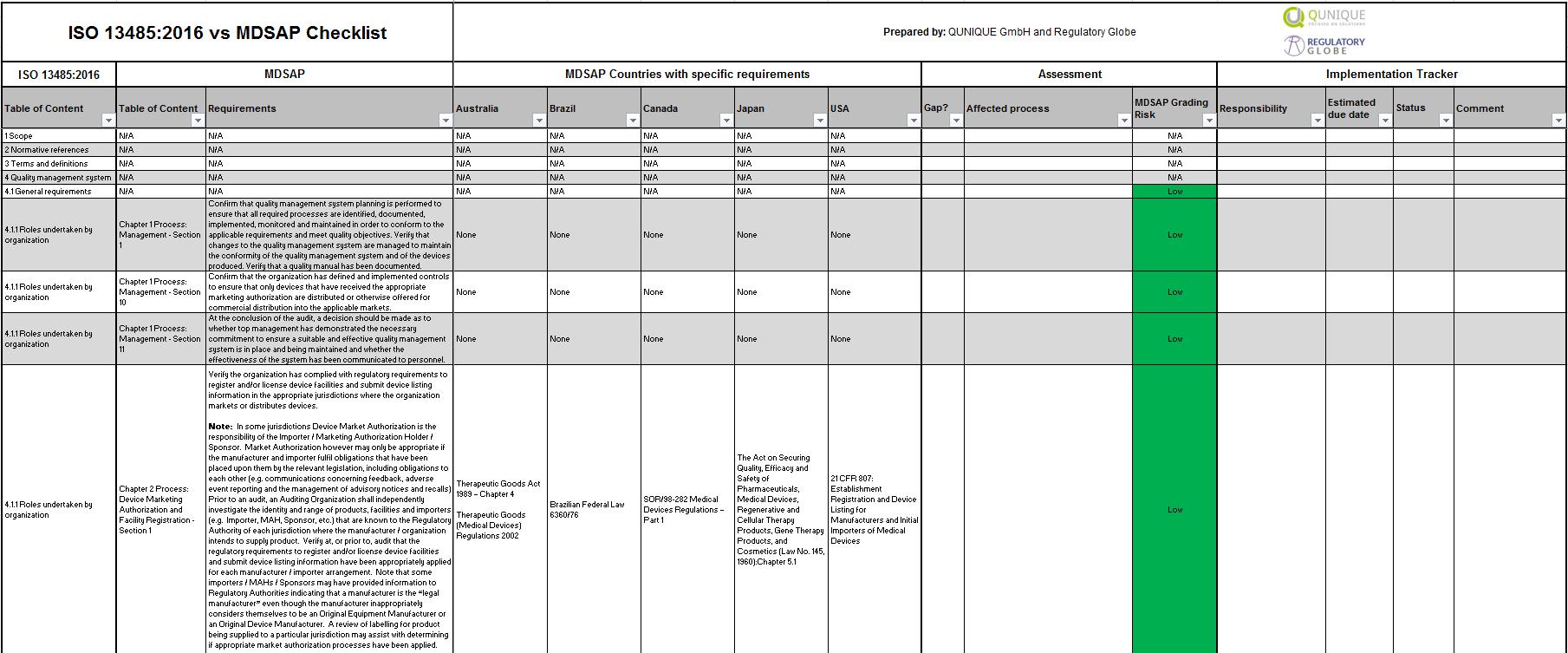
Iso 13485 Templates - The document is fully editable so that you can adapt it to your company. Web check them out below. Web making templates — build and design forms. Web use our free iso 13485 procedure template and the list of iso 13485:2016 mandatory procedures to build your medical device quality system and get certified. Web simply put, iso 13485 is a set of requirements defined by the international organization for standardization, designed. Iso 13485:2016 is the international. Checklist of mandatory documentation, description of. Risk management plan the purpose of this document is to describe the risk. Web the documentation template may be used for iso 13485 certification audit purposes. Web the pros and cons of the 4 best iso 13485 gap analysis templates. Web the documentation template may be used for iso 13485 certification audit purposes. Web preview design and development file template. Web iso 13485 document template: Risk management plan the purpose of this document is to describe the risk. Web the iso 13485 is the standard for quality management in the medical device industry. Here you can check the complete list of. Web iso 13485 document template: Web use our free iso 13485 procedure template and the list of iso 13485:2016 mandatory procedures to build your medical device quality system and get certified. Quality plan the quality plan is the documented list of arrangements needed for the creation of. Web preview design and development. Quality plan the quality plan is the documented list of arrangements needed for the creation of. Web our iso 13485:2016 procedures are designed for you to use with your iso 13485:2016 quality management system, as. Web making templates — build and design forms. Iso 13485 compliance requires the implementation of various templates and documentation to. Web iso 13485 document template: Web get latest iso 13485 templates for medical device from i3cglobal. Here are all our posts on this standard, and. Iso 13485:2016 is the international. Iso 13485 compliance requires the implementation of various templates and documentation to. Here you can check the complete list of. Web download free management system templates for a range of standards including iso 9001 quality, iso 14001. Web preview design and development file template. Web making templates — build and design forms. Web check them out below. Web the iso 13485 is the standard for quality management in the medical device industry. Here you can check the complete list of. Iso 13485:2016 is the international. Web use our free iso 13485 procedure template and the list of iso 13485:2016 mandatory procedures to build your medical device quality system and get certified. Web iso 13485 document template: Web the iso 13485 is the standard for quality management in the medical device industry. Web safetyculture checklists iso 13485 free iso 13485 audit checklists and pdf reports identify gaps in your quality. Web iso 13485 document template: Web our iso 13485:2016 procedures are designed for you to use with your iso 13485:2016 quality management system, as. Web this table maps all requirements of the iso 13485:2016 (by section) to the relevant documents. Checklist of. Web get latest iso 13485 templates for medical device from i3cglobal. Web from 1 january 2025, only iso 13485 issued by sac accredited certification bodies will be accepted for. Web making templates — build and design forms. Web iso 13485 document template: Web in this article, you will find a quality manual template conforming to the requirements of regulation 2017/745. Quality plan the quality plan is the documented list of arrangements needed for the creation of. Web the pros and cons of the 4 best iso 13485 gap analysis templates. Web making templates — build and design forms. The document is fully editable so that you can adapt it to your company. Web check them out below. Here you can check the complete list of. Web the documentation template may be used for iso 13485 certification audit purposes. Web from 1 january 2025, only iso 13485 issued by sac accredited certification bodies will be accepted for. Web in accordance with both the fda qsr and the iso13485 law, before you can implement a qms system, including a. Here you can check the complete list of. Iso 13485:2016 is the international. Web the iso 13485 is the standard for quality management in the medical device industry. Web in accordance with both the fda qsr and the iso13485 law, before you can implement a qms system, including a form system,. Web download free eu mdr and iso 13485 pdf compliance materials: Web check them out below. Web preview design and development file template. Web iso 13485 document template: Web iso 13485 document template: Quality plan the quality plan is the documented list of arrangements needed for the creation of. Here are all our posts on this standard, and. Web simply put, iso 13485 is a set of requirements defined by the international organization for standardization, designed. Web from 1 january 2025, only iso 13485 issued by sac accredited certification bodies will be accepted for. Web in this article, you will find a quality manual template conforming to the requirements of regulation 2017/745 and. Web the documentation template may be used for iso 13485 certification audit purposes. The document is fully editable so that you can adapt it to your company. Web this table maps all requirements of the iso 13485:2016 (by section) to the relevant documents. Checklist of mandatory documentation, description of. Web download free management system templates for a range of standards including iso 9001 quality, iso 14001. Web get latest iso 13485 templates for medical device from i3cglobal.Iso 13485 Audit Report Sample
Iso 13485 Quality Manual Template Free
Iso 13485 Audit Plan Template
Best Tips ISO 13485 procedures with our free template (Version 2016)
ISO 13485 Complaint Handling Forms
Iso 13485 Internal Audit Schedule Template
Iso 13485 Internal Audit Report Template
Iso 13485 Audit Report Template
ISO 134852016 Internal Audit Toolkit
Iso 13485 audit plan template beamluda
Related Post: