Master Manufacturing Record Template
Master Manufacturing Record Template - Web the master manufacturing record must include: Master manufacturing record means, for each project, the template document proposed. (a) the name of the dietary supplement to be manufactured. Web whereas a master production record is a template, the batch production record must be used to record production and qa. Web i am looking for templates of master production record (per 21 cfr part 211.186), and master batch record (per 21. An accurate reproduction of the master batch record. ( a) the name of the dietary supplement to be manufactured and the. Web click batch record in the main menu and then click on the master production record menu. Web this report puts their name in the manufacturing industry. With this truth, a manufacturing report should be well. (a) the name of the dietary supplement to be manufactured. Master manufacturing record means, for each project, the template document proposed. Web this master manufacturing record (mmr) template for cbd tinctures contains all 21 cfr part 111 required elements for a. Web click batch record in the main menu and then click on the master production record menu. Web oracle. Web oracle manufacturing execution system (mes) for process manufacturing provides comprehensive electronic master batch. I was just making a template for master. Web in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features. Web click batch record in the main menu and then click on the master production record menu.. Web a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. To understand how to setup a master production record to understand the. I was just making a template for master. Web standard operating procedure (sop) for preparation, review, approval, issuance, maintenance, and archival of. Click the add new record. An accurate reproduction of the master batch record. These are used to document information. (a) the name of the dietary supplement to be manufactured. Web a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. Web the master manufacturing record must include: (a) the name of the dietary supplement to be manufactured. Click the add new record. I was just making a template for master. Web click batch record in the main menu and then click on the master production record menu. These are used to document information. Web the master manufacturing record must include: I was just making a template for master. Web caliberbrm’s master batch record (mbr) is configurable for any product and batch size (fixed and variable). An accurate reproduction of the master batch record. Web standard operating procedure (sop) for preparation, review, approval, issuance, maintenance, and archival of. Web how to prepare a batch production record (with template) february 22, 2022 from caroline o'donnell cheep. The mfr is a set of instructions so tells you to produce a product batch, while the bmr. Web this report puts their name in the manufacturing industry. Web master production records and batch production records have several professional aliases. Master manufacturing record. Web standard operating procedure (sop) for preparation, review, approval, issuance, maintenance, and archival of. Web a master batch record (mbr), also known as a master production record (mpr) is a document that contains the. I was just making a template for master. Web i am looking for templates of master production record (per 21 cfr part 211.186), and master batch. Web a batch manufacturing record, or bmr, is a document containing the details of the manufacture of each product batch, across. The mfr is a set of instructions so tells you to produce a product batch, while the bmr. Web the master manufacturing record must include: Web the master manufacturing record must include: Web standard operating procedure (sop) for preparation,. Web master production records and batch production records have several professional aliases. With this truth, a manufacturing report should be well. I was just making a template for master. Web a batch manufacturing record, or bmr, is a document containing the details of the manufacture of each product batch, across. Click the add new record. Web suitable for any size manufacturer, this template will enable you to track every detail from the moment you receive an order. Web click batch record in the main menu and then click on the master production record menu. Web i am looking for templates of master production record (per 21 cfr part 211.186), and master batch record (per 21. (a) the name of the dietary supplement to be manufactured. (a) the name of the dietary supplement to be manufactured and the. Web oracle manufacturing execution system (mes) for process manufacturing provides comprehensive electronic master batch. These are used to document information. Web caliberbrm’s master batch record (mbr) is configurable for any product and batch size (fixed and variable). Web master production records are essentially written instructions for a specific manufacturing process, and the fda requires a different master production record. Web master production records and batch production records have several professional aliases. Web this master manufacturing record (mmr) template for cbd tinctures contains all 21 cfr part 111 required elements for a. An accurate reproduction of the master batch record. Web the master manufacturing record must include: Web this report puts their name in the manufacturing industry. Web the master manufacturing record must include: Web in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features. Web whereas a master production record is a template, the batch production record must be used to record production and qa. Web standard operating procedure (sop) for preparation, review, approval, issuance, maintenance, and archival of. ( a) the name of the dietary supplement to be manufactured and the. Master manufacturing record means, for each project, the template document proposed.PHARMACEUTICAL BATCH MANUFACTURING RECORD Sample Download M A N O X
Master Production Record Feature from InstantGMP
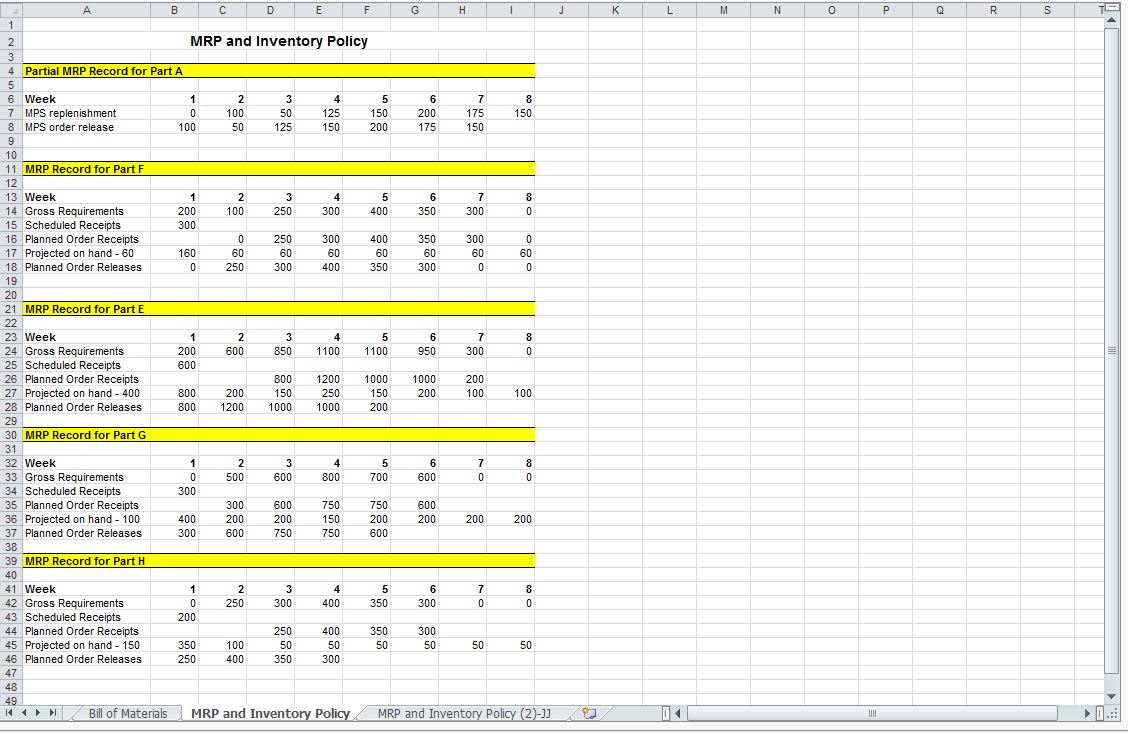
Solved Complete the Master Production Schedule (MPS) record
Master Production Schedule Master Production Template
Master Production Record Feature from InstantGMP
Great Master Production Schedule Excel Spreadsheet Job Application
Batch records are a critical part of maintaining Good Manufacturing
Pharmaceutical Batch Manufacturing Record Template
Mfr
Batch Manufacturing Record (BMR) Pharmaceutical Manufacturing M A N
Related Post: