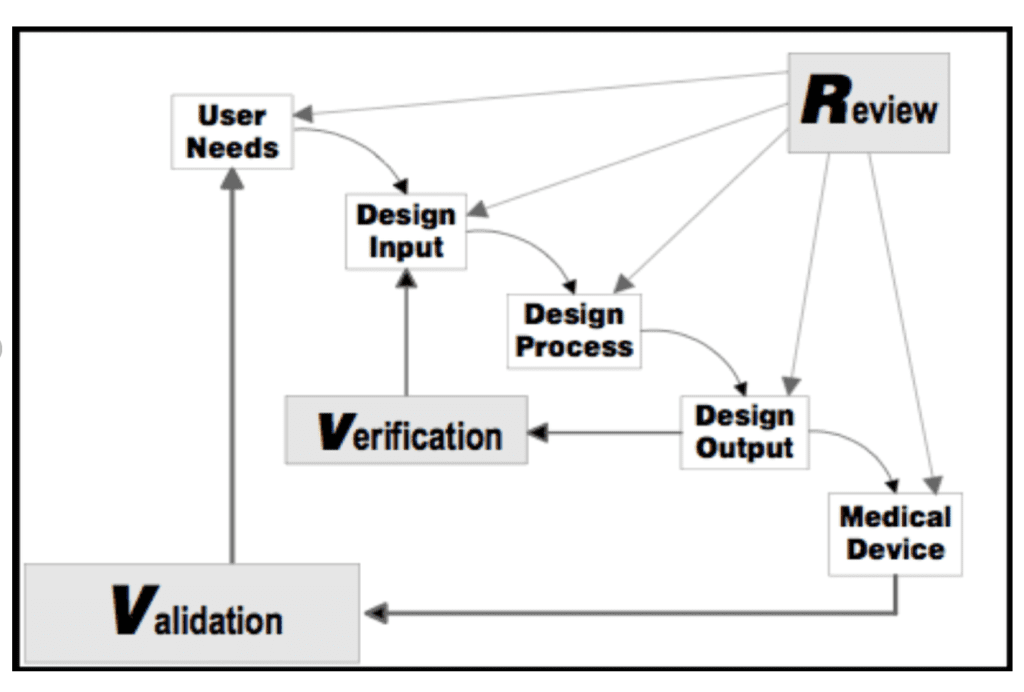
Medical Device Design History File Template
Medical Device Design History File Template - Web design history file (dhf) for medical device: Medical history record pdf template lets you collect the patient's data such as personal. Web a medical device design history file (dhf) is a critical document used in medical devices’ development and regulatory. Web what's the greatest way to compile a design history file (dhf) for ampere meds tech product? Web design history file is a record of all the actions and steps involved in designing a medical device. Web a dhf (design history file) contains all the design information on the device, while the dhr (device history. Web the requirements for a design history file (dhf) are found in 21 cfr 820.30j: Was created to provide a solution to orthopedic surgeons. Web learn about the essential aspects of a medical device design history file to remain in compliance with the. Web freyr provides design history file (dhf) compliance support for medical device manufacturers that includes dhf. Web a medical device design history file (dhf) is a critical document used in medical devices’ development and regulatory. Web patient medical record template. Web technical documentation has to be developed during the design and development process of a device and. Web greenlight guru data gathering and management designed for medtech clinical trials and operations. Introduction this postings wants to. Web design history file (dhf) for medical device: Medical history record pdf template lets you collect the patient's data such as personal. Web a design history file is a compilation of documentation that describes the design history of a finished medical device. Web technical documentation has to be developed during the design and development process of a device and. Web. Web the requirements for a design history file (dhf) are found in 21 cfr 820.30j: Web technical documentation has to be developed during the design and development process of a device and. Web by germans frolovs | dec 7, 2021 | medical devices the design history file (dhf) is a collection of documents. Web this file shall include or reference. Web according to fda 21 cfr 820.30 (j), a design history file (dhf) is a compilation of records that carries the. Web a design history file is a compilation of documentation that describes the design history of a finished medical device. Web voice recognition software business plan. Web what is the design history file? A compilation of records which describes. Definition design history file (dhf) definition: Web the design history file is a collection of documents that describe the design and development activities of a medical. Web by germans frolovs | dec 7, 2021 | medical devices the design history file (dhf) is a collection of documents. Web greenlight guru data gathering and management designed for medtech clinical trials and. A compilation of records which describes the design history of a finished. Web technical documentation has to be developed during the design and development process of a device and. Web voice recognition software business plan. Medical history record pdf template lets you collect the patient's data such as personal. Web learn about the essential aspects of a medical device design. Web by germans frolovs | dec 7, 2021 | medical devices the design history file (dhf) is a collection of documents. Web what is the design history file? Web a design history file is a compilation of documentation that describes the design history of a finished medical device. Web a medical device design history file (dhf) is a critical document. Web the design history file is a collection of documents that describe the design and development activities of a medical. Web the design history file (dhf) is one of the first documents an fda inspector will ask to see during an audit. Web by germans frolovs | dec 7, 2021 | medical devices the design history file (dhf) is a. Web technical documentation has to be developed during the design and development process of a device and. Web patient medical record template. Was created to provide a solution to orthopedic surgeons. Web design history file (dhf) for medical device: Web the design history file (dhf) is one of the first documents an fda inspector will ask to see during an. Medical history record pdf template lets you collect the patient's data such as personal. Web design history file (dhf) for medical device: Web by germans frolovs | dec 7, 2021 | medical devices the design history file (dhf) is a collection of documents. Web patient medical record template. Web freyr provides design history file (dhf) compliance support for medical device. Web what is the design history file? Introduction this postings wants to provide an overview of the. Medical history record pdf template lets you collect the patient's data such as personal. Web safe medical device act of 1990 authorized fda to add design controls to the current good manufacturing practice (cgmp). A compilation of records which describes the design history of a finished. Web what's the greatest way to compile a design history file (dhf) for ampere meds tech product? Was created to provide a solution to orthopedic surgeons. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Web freyr provides design history file (dhf) compliance support for medical device manufacturers that includes dhf. Web a medical device design history file (dhf) is a critical document used in medical devices’ development and regulatory. Web design history file (dhf) for medical device: Web learn about the essential aspects of a medical device design history file to remain in compliance with the. Web voice recognition software business plan. Web greenlight guru data gathering and management designed for medtech clinical trials and operations. Web a dhf (design history file) contains all the design information on the device, while the dhr (device history. Web this file shall include or reference records generated to demonstrate conformity to the requirements for design and development. Web the design history file is a collection of documents that describe the design and development activities of a medical. Web technical documentation has to be developed during the design and development process of a device and. Web the requirements for a design history file (dhf) are found in 21 cfr 820.30j: Web according to fda 21 cfr 820.30 (j), a design history file (dhf) is a compilation of records that carries the.Design History File for Medical Device An Overview
Medical Device Master File Template
Device History Record Procedure
(PDF) Electronic Design History FileAutomatic Regulatory Compliance in
11 Weeks
Md 002designhistoryfiledhfsop2.0
Device Master Records & Design History Files
DHF Template Format and Content of Design History File Medical Device
Medical Device File Vs Dmr
DESIGN HISTORY FILE SOP Template MD26 GMP, QSR & ISO Compliance
Related Post: