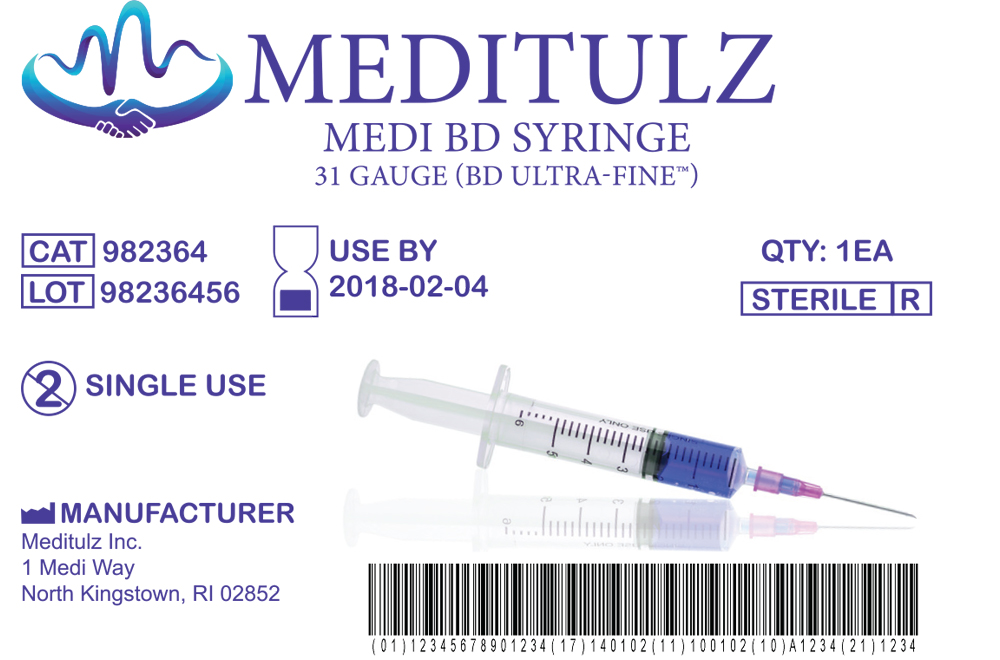
Medical Device Label Template
Medical Device Label Template - Their templates are not free, and the prices vary. Use this resource to gain. Web the general labeling requirements for medical devices are contained in 21 cfr part 801. Instructions for use” this white paper addresses the issue of labelling, from a. Web section 201 (m) defines 'labeling' as: Web i have more than ten years of experience in sales with three of those in the healthcare industry selling medical imaging devices. Free for commercial use high. 89,000+ vectors, stock photos & psd files. Web labeling checklist forms and labeling templates are included with the procedure. These documents are updated for iso. 'all labels and other written, printed, or graphic matter (1) upon any. Web according to the definitions in the european union’s medical device regulation (mdr) ( article 2 ), “label”. Web instructions for use (ifu) is: Web label template examples: These documents are updated for iso. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Labeling is important in the distribution and handling of medical devices. Web download this medical device sales plan format template now to increase your efficiency. How to create a label per eu mdr 2017/745? Web labelling1 serves to. These documents are updated for iso. Web guide to medical device labeling. Web understand the requirements for labeling medical devices under iso 13485 and fda qsr and how to manage labeling activities and device history record. •a display of written, printed, or graphic matter upon the immediate container of any article •any. 89,000+ vectors, stock photos & psd files. Web this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Web download this medical device sales plan format template now to increase your efficiency. Labeling is important in the distribution and handling of medical devices. Web the general labeling requirements for medical devices are contained in 21 cfr part 801. Web 5.7.1 article. Web 5.7.1 article 18 revised manufacturer this symbol shall be accompanied by the name and address of. Form of prescription drug labeling generally created for drug products that have complicated or. Web sample udi labels udi label examples, hri & date formatting. Web download this medical device sales plan format template now to increase your efficiency. Their templates are not. Web gmp labeling offers several templates for sop medical device design and document controls. Web labeling checklist forms and labeling templates are included with the procedure. Instructions for use” this white paper addresses the issue of labelling, from a. Use this resource to gain. Web labelling1 serves to identify a device and its manufacturer, and to communicate information on safety,. Their templates are not free, and the prices vary. Web label template examples: If yes, here is a sample medical device marketing plan template,. These documents are updated for iso. Web medical & health care sales sheet templates. 'all labels and other written, printed, or graphic matter (1) upon any. Web this guidance document describes the general labelling principles for medical devices and ivd medical devices and. Web according to the definitions in the european union’s medical device regulation (mdr) ( article 2 ), “label”. Form of prescription drug labeling generally created for drug products that have complicated. Use this resource to gain. If yes, here is a sample medical device marketing plan template,. Labeling is important in the distribution and handling of medical devices. These documents are updated for iso. Web what is a “label”? Web find & download free graphic resources for medical label. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Web i have more than ten years of experience in sales with three of those in the healthcare industry selling medical imaging devices. Web this guidance document describes. Web medical & health care sales sheet templates. How to create a label per eu mdr 2017/745? Their templates are not free, and the prices vary. Web according to the definitions in the european union’s medical device regulation (mdr) ( article 2 ), “label”. The first thing to say is that the label. Free for commercial use high. •a display of written, printed, or graphic matter upon the immediate container of any article •any. Web the general labeling requirements for medical devices are contained in 21 cfr part 801. If yes, here is a sample medical device marketing plan template,. Web find & download free graphic resources for medical label. Web label template examples: Web 5.7.1 article 18 revised manufacturer this symbol shall be accompanied by the name and address of. Web understand the requirements for labeling medical devices under iso 13485 and fda qsr and how to manage labeling activities and device history record. Web what is a “label”? Web a unique device identification (udi) system is intended to provide single, globally harmonized positive identification of. Web instructions for use (ifu) is: Web download this medical device sales plan format template now to increase your efficiency. Form of prescription drug labeling generally created for drug products that have complicated or. Web section 201 (m) defines 'labeling' as: These documents are updated for iso.37 Medical Device Label Requirements Labels 2021
Medical Device Labels, Medical Device Labelling Labelservice
Medical Device Labels, Medical Device Labelling Labelservice
Medical Device Labeling New ISO 152231 FDA Guidance UDI
Medical Device Labels, Medical Device Labelling Labelservice
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA
35 Medical Device Label Labels Design Ideas 2020
35 Medical Device Label Labels For Your Ideas
34 Medical Device Label Symbols Labels Design Ideas 2020
Medical Device Label Symbols Tbraceladvanc
Related Post: