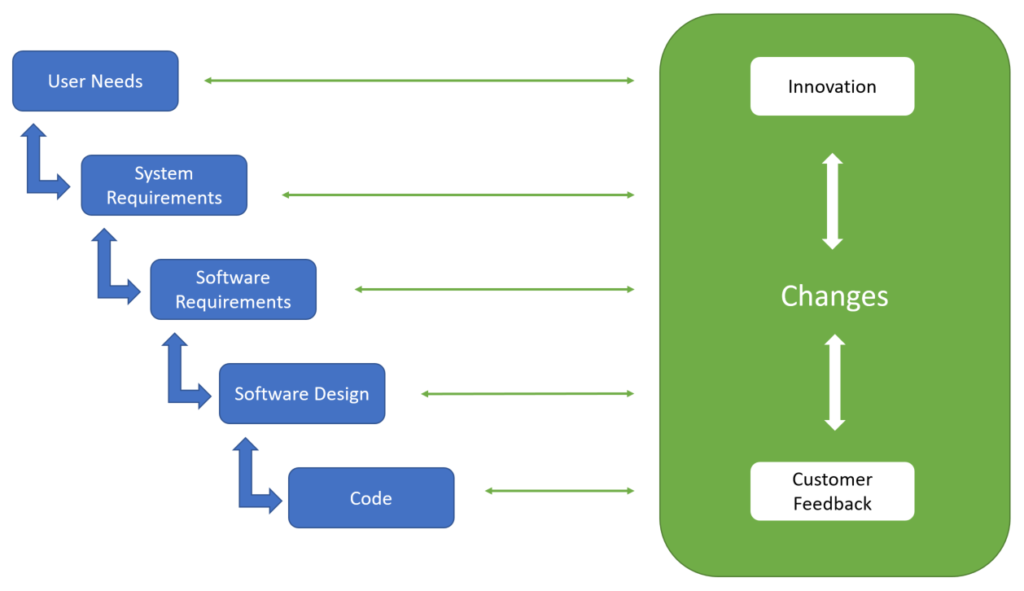
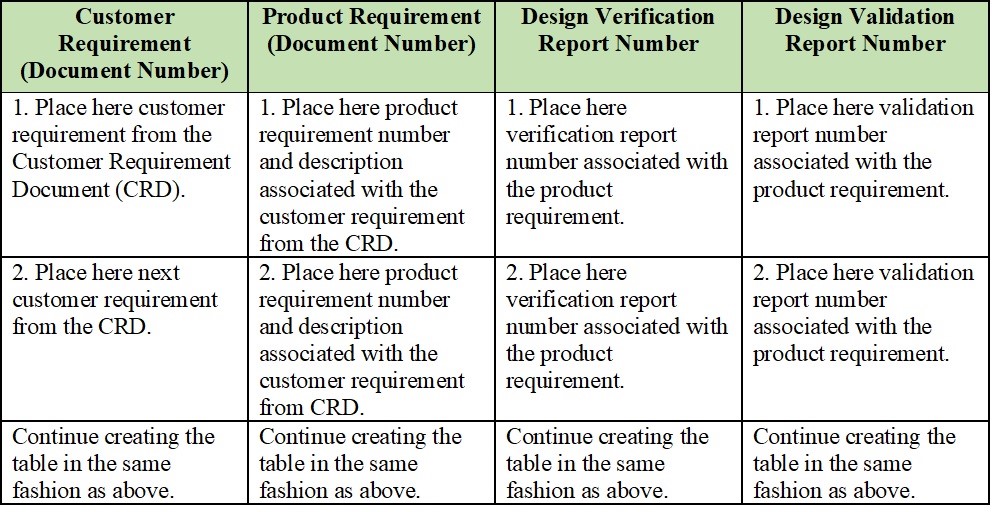
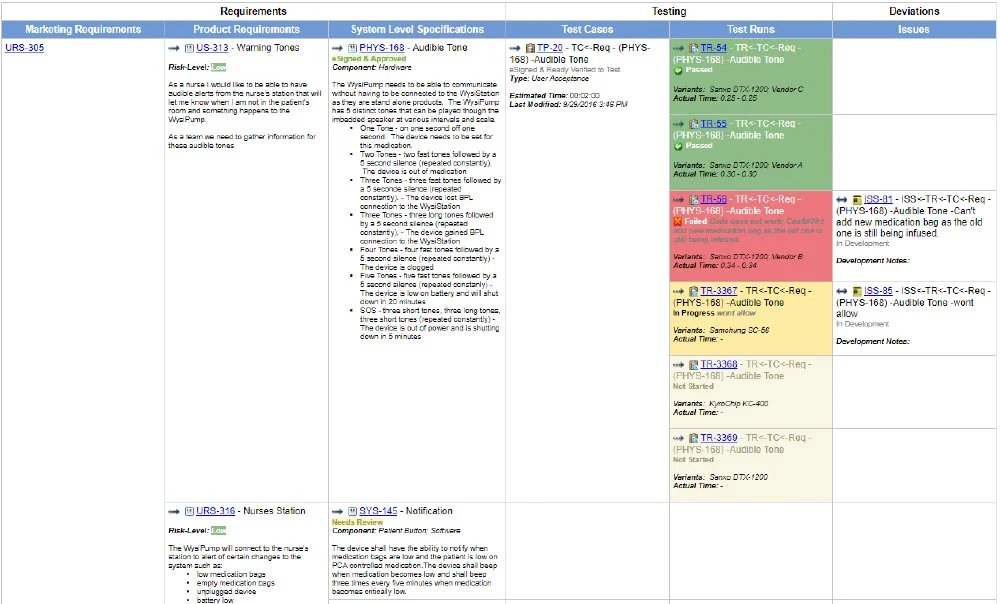
Medical Device Traceability Matrix Template
Medical Device Traceability Matrix Template - Web one way to simplify the process for yourself? Web an complete guide to creating a traceability matrix. Web the first step to build a requirements traceability matrix is to create the template, or shell, of your matrix. Web use this product matrix template for own medical instrument. Web our traceability matrix template is a component of our iso 13485 certified quality management system. A traceability matrix helps you. Web we have a selection of free and premium templates available ranging from checklists to hazard traceability matrixes. Web free template traceability matrix this traceability matrix is an essential component of your design history file (dhf). Web risk management plan template (medical device and iso 14971) risk analysis hazard traceability matrix; This column should contain the id of any associated utilities used for. Web the following elements define a traceability matrix for software as a medical device (samd): Web a traceability matrix is a simple visualization of the linkages between the key areas of design controls, such as. Web requirements traceability matrix associated id(s): The template is fully editable with microsoft excel and. Web use this product matrix template for own medical instrument. Web risk management plan template (medical device and iso 14971) risk analysis hazard traceability matrix; Web a traceability matrix guarantee the detailed translation of user necessarily up actionable simple and regulatory. A traceability matrix helps you. Download the one page excel sheet and open it. Web one way to simplify the process for yourself? Web a traceability matrix is a simple visualization of the linkages between the key areas of design controls, such as. Web our traceability matrix template is a component of our iso 13485 certified quality management system. Web an complete guide to creating a traceability matrix. Medical device traceability can be easy — if you follow the right steps. Web the. Web the following elements define a traceability matrix for software as a medical device (samd): Download the one page excel sheet and open it. Web a traceability matrix is a simple visualization of the linkages between the key areas of design controls, such as. Web an complete guide to creating a traceability matrix. Web risk management plan template (medical device. This column should contain the id of any associated utilities used for. But don’t just bear are word for it. Web the following elements define a traceability matrix for software as a medical device (samd): Learn how the create a requirements traceability matrix template in excel. Web we have a selection of free and premium templates available ranging from checklists. Web we have a selection of free and premium templates available ranging from checklists to hazard traceability matrixes. Learn how the create a requirements traceability matrix template in excel. Web get 5 simple steps to medical device traceability. Web use this product matrix template for own medical instrument. Web #1 hello all, our notified body has requested we provide a. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Web the following elements define a traceability matrix for software as a medical device (samd): A traceability matrix helps you. Web our traceability matrix template is a component of our iso 13485 certified quality management system. Web a. Web a traceability matrix should be created after a medical device concept has been submitted for 501 (k) approval (if need be),. Web one way to simplify the process for yourself? Learn how the create a requirements traceability matrix template in excel. The template is fully editable with microsoft excel and. Web free template traceability matrix this traceability matrix is. Web this is a free requirements traceability matrix in excel.xls and.xlsx. Web how to use the medical tracker excel template. Web a traceability matrix is a simple visualization of the linkages between the key areas of design controls, such as. Web an complete guide to creating a traceability matrix. Web the design traceability matrix is intended to obtain information and. Web risk management plan template (medical device and iso 14971) risk analysis hazard traceability matrix; Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. The template is fully editable with microsoft excel and. Begin by entering your name in the name field on the. This column should. Web free template traceability matrix this traceability matrix is an essential component of your design history file (dhf). Web the first step to build a requirements traceability matrix is to create the template, or shell, of your matrix. Web how to use the medical tracker excel template. Web an complete guide to creating a traceability matrix. Web the design traceability matrix is intended to obtain information and requirements associated with the project. Medical device traceability can be easy — if you follow the right steps. Download the one page excel sheet and open it. But don’t just bear are word for it. Web one way to simplify the process for yourself? Web a traceability matrix should be created after a medical device concept has been submitted for 501 (k) approval (if need be),. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Web #1 hello all, our notified body has requested we provide a traceability matrix with detailed verbiage of. Begin by entering your name in the name field on the. A traceability matrix helps you. Web a traceability matrix guarantee the detailed translation of user necessarily up actionable simple and regulatory. Web requirements traceability matrix associated id(s): Web get 5 simple steps to medical device traceability. Web our traceability matrix template is a component of our iso 13485 certified quality management system. The template is fully editable with microsoft excel and. This column should contain the id of any associated utilities used for.Traceability Matrix Webinar for 510k Software Documentation Medical
How to Make Design Controls More Efficient With Traceability Matrix
FDA Expectations for Traceability in Device & Diagnostic Design
What Is a Requirements Traceability Matrix? Your AZ Guide Perforce
Developing a Med Device Traceability Matrix EMMA International
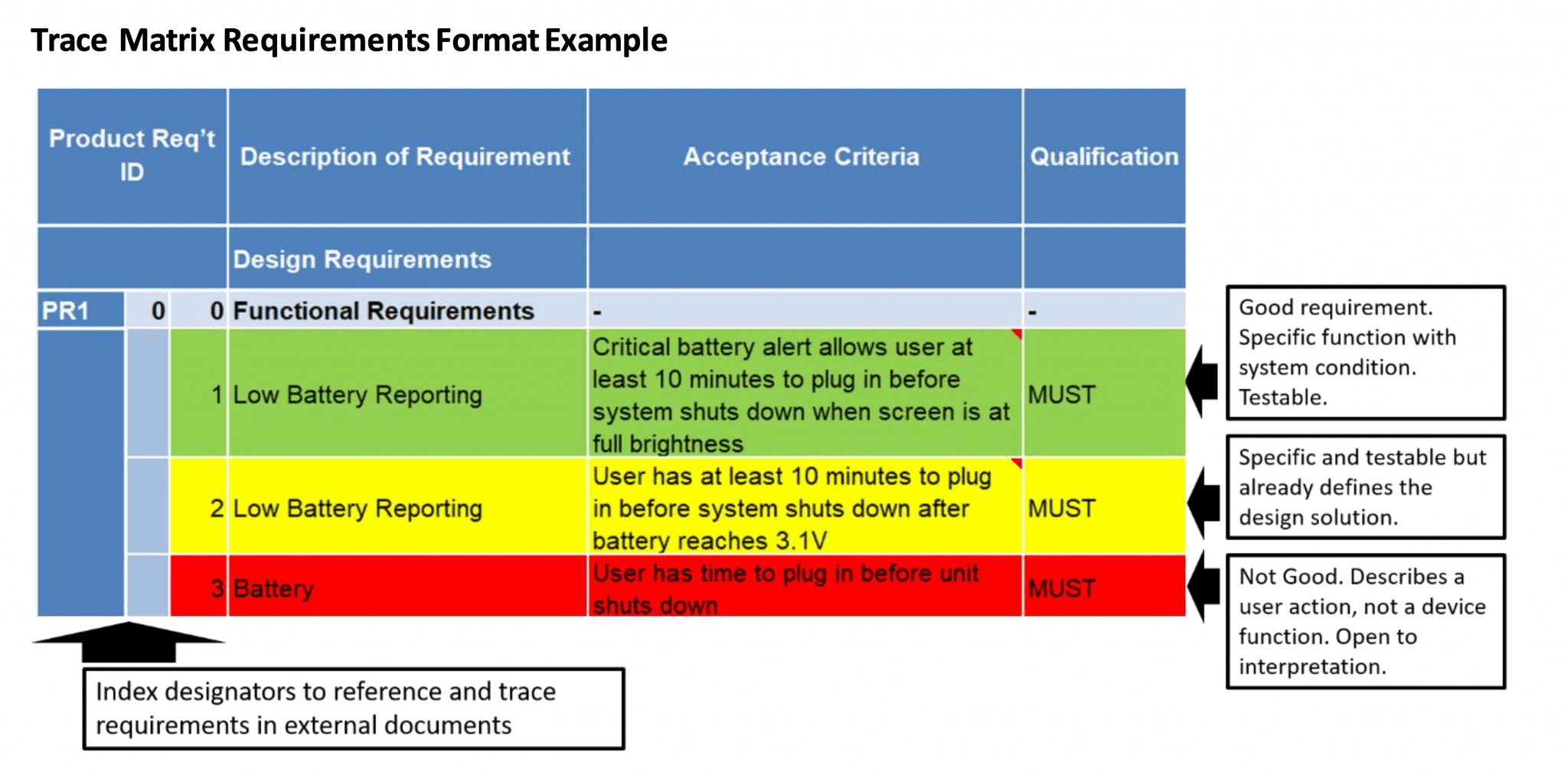
How to Define Product Requirements for Medical Devices Simbex
Traceability Matrix PDF Call Centre Computing
Medical device risk assessment template generatordast
Medical Device Design Insights and Resources
Project Traceability Matrix Template 02/2022
Related Post: