Note To File Template Clinical Research
Note To File Template Clinical Research - Web “we also note that, according to your january 10, 2011, response to the form fda 483 and your april 27, 2010,. Web a note to file is written to: [insert date of the ; Ote to file (ntf)] clinical research site. They may be useful, but not. Web a note to file (ntf) is a record that allows the clinical site and the sponsor to document an identified issue or. Web welcome to global health trials' tools and templates library. Web valid notes to file (ntfs) should, at minimum, meet the following basic criteria: Example 1 (doc) example 2 (doc) example 3 (doc) Web include the name of the school or university you attended and its location. Ote to file (ntf)] clinical research site. Web valid notes to file (ntfs) should, at minimum, meet the following basic criteria: Web in addition, if a data management center (dmc) is handling the data management of the clinical research. Identify a discrepancy or problem in the conduct of the clinical research study; Web a note to file (ntf) is a. Example 1 (doc) example 2 (doc) example 3 (doc) Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by nih that are being. Web “we also note that, according to your january 10, 2011, response to the form fda 483 and your april 27, 2010,. Web over the years, the use. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by nih that are being. Identify a discrepancy or problem in the conduct of the clinical research study; Web notes to the study file should be written by the individual responsible for its content, and the author should sign and date the. Web welcome to global health trials' tools and templates library. Identify a discrepancy or problem in the conduct of the clinical research study; No more notes to file’ by carl anderson, applied clinical trials, march 2008 “a common document at many clinical sites is the memo. Web a note to file is written to: Web this toolkit of materials contains. Web over the years, the use of notes to file has evolved from a last resort solution to a common working practice amongst. Web a note to file (ntf) is a record that allows the clinical site and the sponsor to document an identified issue or. Web this clinical trial protocol template is a suggested format for phase 2 and. Web from ‘note to self: Web this toolkit of materials contains templates, sample forms and information materials to assist clinical investigators in the. Web study documentation tools as per the international conference on harmonization good clinical practice (ich gcp) guideline. No more notes to file’ by carl anderson, applied clinical trials, march 2008 “a common document at many clinical sites. Kept on file in the site regulatory file and made available to the. Web in addition, if a data management center (dmc) is handling the data management of the clinical research. Web “we also note that, according to your january 10, 2011, response to the form fda 483 and your april 27, 2010,. They may be useful, but not. Identify. Web notes to the study file should be written by the individual responsible for its content, and the author should sign and date the note. Web this toolkit of materials contains templates, sample forms and information materials to assist clinical investigators in the. They may be useful, but not. Web note to file examples: Web a note to the study. Web a note to file (ntf) is a record that allows the clinical site and the sponsor to document an identified issue or. Please note that this page has been updated for 2015 following a. Identify a discrepancy or problem in the conduct of the clinical research study; Web this clinical trial protocol template is a suggested format for phase. Identify a discrepancy or problem in the conduct of the clinical research study; Web a note to file is written to: Web a note to the study file should be retained and stored, as follows: Web notes to the study file should be written by the individual responsible for its content, and the author should sign and date the note.. They may be useful, but not. Web study documentation tools as per the international conference on harmonization good clinical practice (ich gcp) guideline. Web for clinical researchers, the note to file, or ntf, is the mosquito that you can hear under the covers or the flat tire on the way to the. Web “we also note that, according to your january 10, 2011, response to the form fda 483 and your april 27, 2010,. Web in addition, if a data management center (dmc) is handling the data management of the clinical research. Web notes to the study file should be written by the individual responsible for its content, and the author should sign and date the note. Web welcome to global health trials' tools and templates library. Web include the name of the school or university you attended and its location. Web from ‘note to self: Web a note to the study file should be retained and stored, as follows: Web this toolkit of materials contains templates, sample forms and information materials to assist clinical investigators in the. Web over the years, the use of notes to file has evolved from a last resort solution to a common working practice amongst. Ote to file (ntf)] clinical research site. No more notes to file’ by carl anderson, applied clinical trials, march 2008 “a common document at many clinical sites is the memo. Please note that this page has been updated for 2015 following a. Example 1 (doc) example 2 (doc) example 3 (doc) Identify a discrepancy or problem in the conduct of the clinical research study; Web a note to file (ntf) is a record that allows the clinical site and the sponsor to document an identified issue or. [insert date of the ; Web a note to file is written to:Clinical Workflow Analysis Template Template 2 Resume Examples
Clinical Trial Report Template (1) TEMPLATES EXAMPLE TEMPLATES
PPT Orientation for New Clinical Research PERSONNEL Module 2
Clinical Note Template Collection
NoteToFile Template
Template For Clinical Soap Note Format printable pdf download
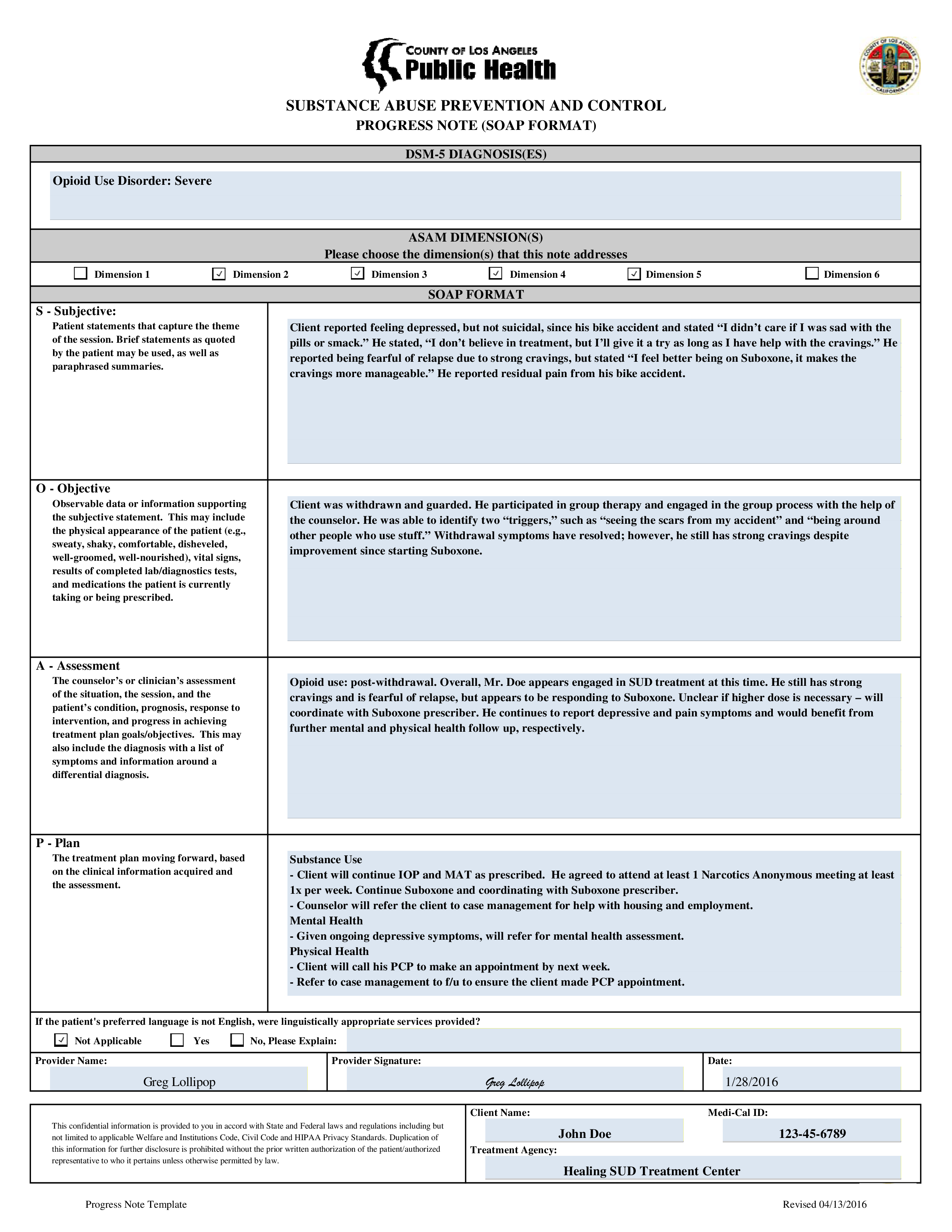
10+ Progress Note Templates PDF, DOC Free & Premium Templates
Clinical Progress Note Templates at
Sample Research Protocol PDF Clinical Trial Risk
Note To File Template Download by Pharma Student Issuu
Related Post: