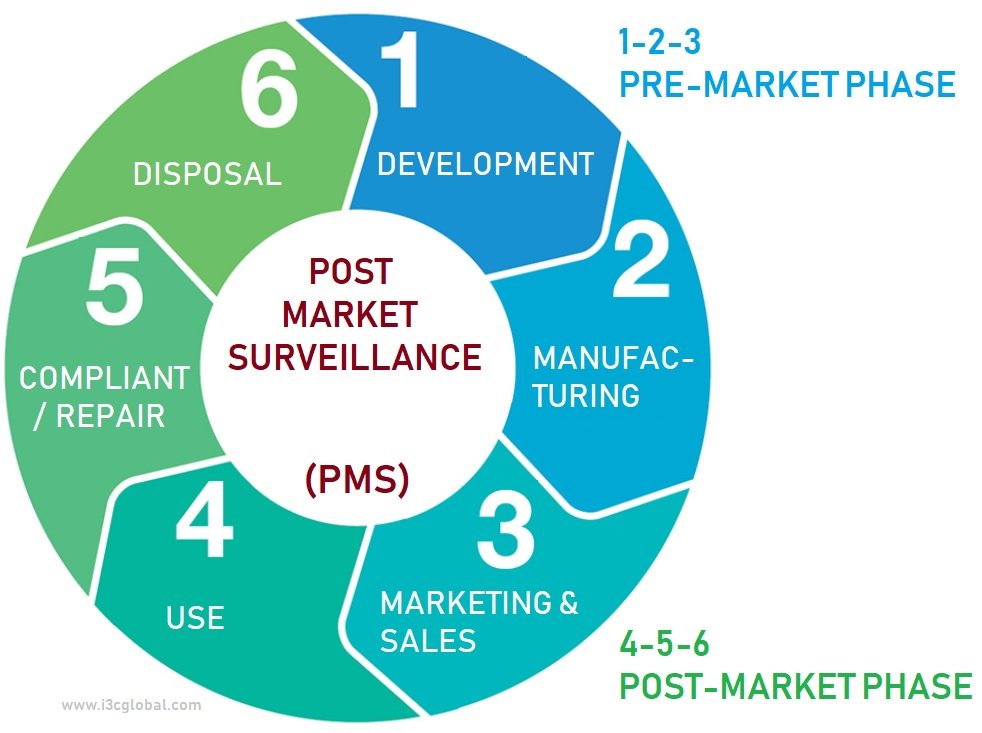
Post Market Surveillance Plan Template
Post Market Surveillance Plan Template - Web iso 13485 document template: Web post market surveillance sop & template criteria for conducting post market surveillance (pms) study as per eu. Web post market surveillance plan. Web post market surveillance (pms) is primarily concerned with establishing the pms plan for the device. Web when formulating the device pms plan, it is pertinent to remember that iso 13485 applies to all medical devices on the market and in the context of this. The document is fully editable so that you can. Complying with the requirements of iso tr 20416 with the post. Web th august 2020 author: The rationale for establishing a. A post market surveillance plan is a systematic plan of the processes and the activities to continuously monitor the safety and the performance of the medical devices released in the market. Web post market surveillance plan. The rationale for establishing a. It includes the concept of a pms plan. A post market surveillance plan is a systematic plan of the processes and the activities to continuously monitor the safety and the performance of the medical devices released in the market. You can download it as word (.docx), pdf, google docs or. The plan identifies the process and frequency of. Web guidance on appropriate surveillance regarding the transitional provisions under article 110 of the ivdr with regard to. It includes the concept of a pms plan. Web post market surveillance sop & template criteria for conducting post market surveillance (pms) study as per eu. The post market surveillance plan is part of. It includes the concept of a pms plan. Web guidance on appropriate surveillance regarding the transitional provisions under article 110 of the ivdr with regard to. A post market surveillance plan is a systematic plan of the processes and the activities to continuously monitor the safety and the performance of the medical devices released in the market. Web the process. Web post market surveillance sop & template criteria for conducting post market surveillance (pms) study as per eu. The post market surveillance plan is part of the technical. Web the process shall allow a correct characterisation of the performance of the devices and shall also allow a comparison. Scroll down for a preview! The rationale for establishing a. Complying with the requirements of iso tr 20416 with the post. Web guidance on appropriate surveillance regarding the transitional provisions under article 110 of the ivdr with regard to. The rationale for establishing a. Web post market surveillance sop & template criteria for conducting post market surveillance (pms) study as per eu. Web when formulating the device pms plan, it. Web as a consequence, the new eu medical device regulation was published; Web the process shall allow a correct characterisation of the performance of the devices and shall also allow a comparison. The rationale for establishing a. Scroll down for a preview! Web post market surveillance (pms) is primarily concerned with establishing the pms plan for the device. The post market surveillance plan is part of the technical. Web post market surveillance sop & template criteria for conducting post market surveillance (pms) study as per eu. A post market surveillance plan is a systematic plan of the processes and the activities to continuously monitor the safety and the performance of the medical devices released in the market. Complying. Web th august 2020 author: Complying with the requirements of iso tr 20416 with the post. Web as a consequence, the new eu medical device regulation was published; The rationale for establishing a. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device. You can download it as word (.docx), pdf, google docs or markdown file. Scroll down for a preview! Web guidance on appropriate surveillance regarding the transitional provisions under article 110 of the ivdr with regard to. Web iso 13485 document template: The plan identifies the process and frequency of. Web as a consequence, the new eu medical device regulation was published; Web post market surveillance (pms) is primarily concerned with establishing the pms plan for the device. Complying with the requirements of iso tr 20416 with the post. Web th august 2020 author: Web iso 13485 document template: Web when formulating the device pms plan, it is pertinent to remember that iso 13485 applies to all medical devices on the market and in the context of this. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device. Web guidance on appropriate surveillance regarding the transitional provisions under article 110 of the ivdr with regard to. The document is fully editable so that you can. You can download it as word (.docx), pdf, google docs or markdown file. Web iso 13485 document template: Web post market surveillance plan. Web the process shall allow a correct characterisation of the performance of the devices and shall also allow a comparison. Web post market surveillance (pms) is primarily concerned with establishing the pms plan for the device. The rationale for establishing a. The post market surveillance plan is part of the technical. Web see also the dedicated page on clinical evaluation. Scroll down for a preview! A post market surveillance plan is a systematic plan of the processes and the activities to continuously monitor the safety and the performance of the medical devices released in the market. The plan identifies the process and frequency of. Complying with the requirements of iso tr 20416 with the post. Web as a consequence, the new eu medical device regulation was published; Web th august 2020 author: It includes the concept of a pms plan. Web post market surveillance sop & template criteria for conducting post market surveillance (pms) study as per eu.Get Our Image of Medical Device Marketing Plan Template Marketing
PostMarket Surveillance Plan Template QualityMedDev
Post Market Surveillance Plan PMS Plan Template
PostMarket Surveillance
postmarket surveillance plan
Surveillance Report Template Glendale Community Regarding Private
Post Market Surveillance Procedure
Post Market Clinical FollowUp (PMCF) Template by Pharmi Med Ltd Issuu
Post Market Surveillance Application Form
Post Market Surveillance Report Template
Related Post: